
Contributions
Abstract: EP365
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Acute leukemia of ambiguous lineage (ALAL) is a rare form of acute leukemia, with a reported incidence of 1-6%, associated with a poor prognosis with median survival of 14.8 to 18 months. The World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues 2016 Revised Edition classifies ALAL as a separate category from acute myeloid leukemia and acute lymphoblastic leukemia.
Treatment generally consists of acute myeloid leukemia (AML) induction, acute lymphoblastic leukemia (ALL) induction or hybrids of AML and ALL induction. Patients with t(9;22) are generally treated with a tyrosine kinase inhibitor. Eligible patients who achieve complete remission after induction are often treated with a consolidative allogeneic hematopoietic stem cell transplant (HSCT). Little evidence is available for the use of novel therapeutics such as antibody-drug conjugates, bispecific T-cell engagers or chimeric antigen receptor T-cells (CAR-T) in ALAL.
Aims
1. To determine the incidence of ALAL in a single center
2. To determine the characteristics of ALAL patients and their disease
3. To review the treatment employed in ALAL patients
4. To report outcomes of ALAL patients
5. To report incidence of specific immunophenotypic markers which may be therapeutic targets in ALAL
Methods
Using the British Columbia Cancer Agency Leukemia Database, patients with diagnosed the an ALAL between 2000 and 2020 were identified. Information including demographics, date of diagnosis, pathology results, treatment employed and survival data from the database was reviewed by two clinical hematologists. If the diagnosis was felt to meet another diagnosis other than ALAL, the patient was excluded from the analysis. Patient sex, age at diagnosis, immunophenotypic markers, first treatment regimen and bone marrow transplant status were quantified. A survival analysis was performed using a Kaplan-Meier analysis.
Results
63 patients were identified as having ALAL. 16 patients were removed from analysis because of the diagnosis meeting another WHO leukemia classification.
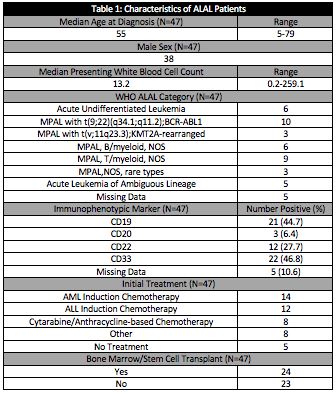
The characteristics of patients, disease and treatment strategies employed for these patients is demonstrated in Table 1. CD33 and CD19 were the most common immunophenotypic targets, with more than 40% of patients being positive, respectively.
The median overall survival was 30.2 months (12.4-48.0 months, CI 95%) with a median follow-up time of 22.1 months. Three-year overall survival was 42.6%. Three-year overall survival was 59.9% and 24.8% in the transplant group and non-transplant group, respectively.
Conclusion
This single-centre, retrospective cohort study offers the first data of ALAL using the updated WHO 2016 criteria for ALALs. That 16 patients were deemed to have alternative diagnoses demonstrates the advances in understanding of acute leukemias and may indicate that the incidence of ALALs is decreasing as more refined diagnostics are used.
MPAL with t(9;22) was the most common subtype of ALAL that was demonstrated. Further review of data is required to determine what percentage of these patients received tyrosine kinase inhibitors. The majority of the patients in our cohort received either an AML or ALL type induction regimens with 51% of the patients going on to receive an allogeneic transplant. This is consistent with previously reported treatment strategies. Most patients had an immunotherapeutic target. This supports the future study of novel immunotherapeutics such as gemtuzumab ozogomicin, blinatumomab, CD19-directed CAR-T products and inotuzumab ozogamicin in ALAL.
Keyword(s): Acute leukemia, Leukemia, Mixed lineage leukemia
Abstract: EP365
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Acute leukemia of ambiguous lineage (ALAL) is a rare form of acute leukemia, with a reported incidence of 1-6%, associated with a poor prognosis with median survival of 14.8 to 18 months. The World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues 2016 Revised Edition classifies ALAL as a separate category from acute myeloid leukemia and acute lymphoblastic leukemia.
Treatment generally consists of acute myeloid leukemia (AML) induction, acute lymphoblastic leukemia (ALL) induction or hybrids of AML and ALL induction. Patients with t(9;22) are generally treated with a tyrosine kinase inhibitor. Eligible patients who achieve complete remission after induction are often treated with a consolidative allogeneic hematopoietic stem cell transplant (HSCT). Little evidence is available for the use of novel therapeutics such as antibody-drug conjugates, bispecific T-cell engagers or chimeric antigen receptor T-cells (CAR-T) in ALAL.
Aims
1. To determine the incidence of ALAL in a single center
2. To determine the characteristics of ALAL patients and their disease
3. To review the treatment employed in ALAL patients
4. To report outcomes of ALAL patients
5. To report incidence of specific immunophenotypic markers which may be therapeutic targets in ALAL
Methods
Using the British Columbia Cancer Agency Leukemia Database, patients with diagnosed the an ALAL between 2000 and 2020 were identified. Information including demographics, date of diagnosis, pathology results, treatment employed and survival data from the database was reviewed by two clinical hematologists. If the diagnosis was felt to meet another diagnosis other than ALAL, the patient was excluded from the analysis. Patient sex, age at diagnosis, immunophenotypic markers, first treatment regimen and bone marrow transplant status were quantified. A survival analysis was performed using a Kaplan-Meier analysis.
Results
63 patients were identified as having ALAL. 16 patients were removed from analysis because of the diagnosis meeting another WHO leukemia classification.
The characteristics of patients, disease and treatment strategies employed for these patients is demonstrated in Table 1. CD33 and CD19 were the most common immunophenotypic targets, with more than 40% of patients being positive, respectively.
The median overall survival was 30.2 months (12.4-48.0 months, CI 95%) with a median follow-up time of 22.1 months. Three-year overall survival was 42.6%. Three-year overall survival was 59.9% and 24.8% in the transplant group and non-transplant group, respectively.

Conclusion
This single-centre, retrospective cohort study offers the first data of ALAL using the updated WHO 2016 criteria for ALALs. That 16 patients were deemed to have alternative diagnoses demonstrates the advances in understanding of acute leukemias and may indicate that the incidence of ALALs is decreasing as more refined diagnostics are used.
MPAL with t(9;22) was the most common subtype of ALAL that was demonstrated. Further review of data is required to determine what percentage of these patients received tyrosine kinase inhibitors. The majority of the patients in our cohort received either an AML or ALL type induction regimens with 51% of the patients going on to receive an allogeneic transplant. This is consistent with previously reported treatment strategies. Most patients had an immunotherapeutic target. This supports the future study of novel immunotherapeutics such as gemtuzumab ozogomicin, blinatumomab, CD19-directed CAR-T products and inotuzumab ozogamicin in ALAL.
Keyword(s): Acute leukemia, Leukemia, Mixed lineage leukemia


