
Contributions
Abstract: EP364
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Extramedullary involvement of B-cell acute lymphoblastic leukemia (EM-ALL) is a rare occurrence, characterized by dismal outcome and the lack of a defined and shared therapeutic approach. Inotuzumab ozogamicin (InO) is a humanized anti-CD22 monoclonal antibody conjugated to calicheamicin. It was developed as a targeted therapy for B-cell malignancies.
Aims
To characterize a series of relapsed/refractory (r/r) adult EM-ALL patients (pts) and evaluate outcome after InO treatment.
Methods
We studied 17 r/r pts (median age, 31 years; range, 19-68 years), who were treated with InO between 2015 and 2020 within a compassionate use program (n=7) or in-label after EMA approval (n=10). Three of the 17 pts were previously treated with tyrosine kinase inhibitors for chronic myeloid leukemia and progressed to blast crisis of B-lymphoid lineage. Bone marrow evaluation and immunophenotyping by flow cytometry revealed acute B-lymphoblastic leukemia in all three pts. All 17 pts were CD22 positive at relapse/progressive disease. Up to six InO cycles (≤2 cycles, n=11; 3-4 cycles, n=4; 5-6 cycles, n=2) were administered according to the previously approved regimen. EM response assessment was performed by CT or PET-CT.
Results
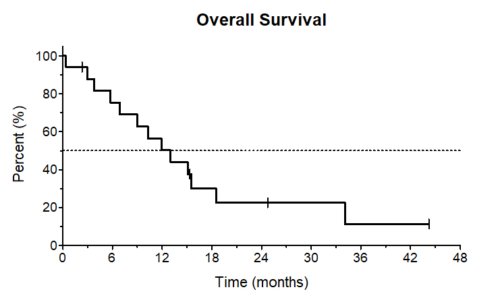
Localization of EM disease included lymph nodes (in total, n=9; including gastric and skin; bone and skin; lung, bone and skin; hepatic and bone; hepatic, n=1 in each of these combinations), bones (n=4), kidney (n=1), peripheral nerves (n=1), pancreas and bone (n=1), and ovary (n=1). At the time of r/r EM-ALL median white blood cell and platelet counts were 4.9/nl (range, 0.7-10.3/nl) and 94.5/nl (range, 11-515/nl), respectively. Eleven pts (49%) were female; ECOG was ≤ 2 in all pts. Cytogenetic analysis at the time of r/r EM-ALL was available in five pts. One patient had a normal karyotype, two were complex and two pts displayed a t(9;22). Complete remission assessed by PET-CT (CR; including EM and hematological/bone marrow CR) after the first InO cycle was achieved in 7 pts (41%), 7 pts had a partial remission (PR) (41%), one (6%) had stable disease (SD) and one patient (6%) died at day 11 of the first InO cycle due to cerebral hemorrhage. One patient had no response assessment after the first induction cycle (6%). After two InO cycles, overall response rate was 94% (n=16), including CR in 9 pts (56%) and PR in six (38%); the patient with SD did not receive further InO treatment (6%). Median follow-up was 12.1 months (range, 0.4-44.9 months) and median overall survival (OS) 11.9 months (95%>CI, 8-20 months; Figure 1). One-year and two-years OS rates were 50% (95%>CI, 25-71%) and 23% (95%>CI, 6-46%), respectively. Age above 60 years had no impact on OS (p=0.48). Seven pts proceeded to allogeneic hematopoietic stem cell transplantation (allo-SCT; CR, PR, n=3, each; progressive disease, n=1). Prior to allo-SCT four pts received ≤2 cycles InO and three pts ≤4 cycles. No occurrence of veno-occlusive disease was reported. Relapse/progressive disease occurred in nine pts and all pts succumbed of their disease. Cumulative incidence of relapse after 12 months was 38%. One patient experienced a molecular relapse, which could be successfully treated with InO again. Four pts died in remission and four pts are in ongoing CR including the patient with prior molecular relapse and InO re-exposure.
Conclusion
This outcome analysis demonstrates that treatment with InO is an effective and promising approach including a successful bridge-to-transplant strategy in this rare subgroup of patients with r/r EM-ALL and abysmal prognosis.
Keyword(s): Antibody response, Extramedullary hematopoiesis, Outcome measurement, Relapsed acute lymphoblastic leukemia
Abstract: EP364
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Extramedullary involvement of B-cell acute lymphoblastic leukemia (EM-ALL) is a rare occurrence, characterized by dismal outcome and the lack of a defined and shared therapeutic approach. Inotuzumab ozogamicin (InO) is a humanized anti-CD22 monoclonal antibody conjugated to calicheamicin. It was developed as a targeted therapy for B-cell malignancies.
Aims
To characterize a series of relapsed/refractory (r/r) adult EM-ALL patients (pts) and evaluate outcome after InO treatment.
Methods
We studied 17 r/r pts (median age, 31 years; range, 19-68 years), who were treated with InO between 2015 and 2020 within a compassionate use program (n=7) or in-label after EMA approval (n=10). Three of the 17 pts were previously treated with tyrosine kinase inhibitors for chronic myeloid leukemia and progressed to blast crisis of B-lymphoid lineage. Bone marrow evaluation and immunophenotyping by flow cytometry revealed acute B-lymphoblastic leukemia in all three pts. All 17 pts were CD22 positive at relapse/progressive disease. Up to six InO cycles (≤2 cycles, n=11; 3-4 cycles, n=4; 5-6 cycles, n=2) were administered according to the previously approved regimen. EM response assessment was performed by CT or PET-CT.
Results
Localization of EM disease included lymph nodes (in total, n=9; including gastric and skin; bone and skin; lung, bone and skin; hepatic and bone; hepatic, n=1 in each of these combinations), bones (n=4), kidney (n=1), peripheral nerves (n=1), pancreas and bone (n=1), and ovary (n=1). At the time of r/r EM-ALL median white blood cell and platelet counts were 4.9/nl (range, 0.7-10.3/nl) and 94.5/nl (range, 11-515/nl), respectively. Eleven pts (49%) were female; ECOG was ≤ 2 in all pts. Cytogenetic analysis at the time of r/r EM-ALL was available in five pts. One patient had a normal karyotype, two were complex and two pts displayed a t(9;22). Complete remission assessed by PET-CT (CR; including EM and hematological/bone marrow CR) after the first InO cycle was achieved in 7 pts (41%), 7 pts had a partial remission (PR) (41%), one (6%) had stable disease (SD) and one patient (6%) died at day 11 of the first InO cycle due to cerebral hemorrhage. One patient had no response assessment after the first induction cycle (6%). After two InO cycles, overall response rate was 94% (n=16), including CR in 9 pts (56%) and PR in six (38%); the patient with SD did not receive further InO treatment (6%). Median follow-up was 12.1 months (range, 0.4-44.9 months) and median overall survival (OS) 11.9 months (95%>CI, 8-20 months; Figure 1). One-year and two-years OS rates were 50% (95%>CI, 25-71%) and 23% (95%>CI, 6-46%), respectively. Age above 60 years had no impact on OS (p=0.48). Seven pts proceeded to allogeneic hematopoietic stem cell transplantation (allo-SCT; CR, PR, n=3, each; progressive disease, n=1). Prior to allo-SCT four pts received ≤2 cycles InO and three pts ≤4 cycles. No occurrence of veno-occlusive disease was reported. Relapse/progressive disease occurred in nine pts and all pts succumbed of their disease. Cumulative incidence of relapse after 12 months was 38%. One patient experienced a molecular relapse, which could be successfully treated with InO again. Four pts died in remission and four pts are in ongoing CR including the patient with prior molecular relapse and InO re-exposure.

Conclusion
This outcome analysis demonstrates that treatment with InO is an effective and promising approach including a successful bridge-to-transplant strategy in this rare subgroup of patients with r/r EM-ALL and abysmal prognosis.
Keyword(s): Antibody response, Extramedullary hematopoiesis, Outcome measurement, Relapsed acute lymphoblastic leukemia


