
Contributions
Abstract: EP360
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Since 2009 RALL-study group has conducted two clinical multicenter trials on the treatment of adult patients with Ph-negative ALL. The majority of adult ALL studies are highly intensive with substantial numbers of allogeneic HSCT in 1CR and tailor their treatment according to MRD levels after the induction phase. RALL studies were based on the non-intensive, but non-interruptive approach without high dose consolidations and without many allo-HSCTs but with autologous HSCT for T-cell ALL as late consolidation. Pregnant women were included in both trials. MRD measurement was carried out only in the second RALL-2016 study.
Aims
to present the comparison of long-term results of both studies and evaluate the impact of MRD measurement in RALL-2016.
Methods
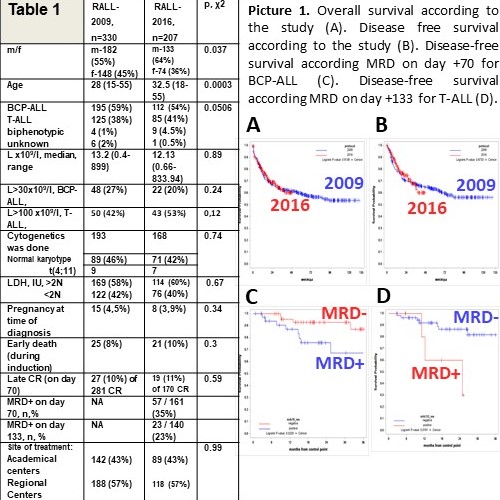
From Jan, 2009, till Dec, 2016, 30 centers had enrolled 330 and since Dec 2016 till Jan 2021, 10 centers included 207 acute Ph-negative lymphoblastic leukemia patients. The main trials characteristics are presented in Table 1. There were no difference between two trials in numbers of autologous HSCTs for T-ALL (42/125 (33%) pts in RALL-2009 and 23/85 (27%) in RALL-2016); no difference in numbers of allo-HSCTs in 1CR (19/281 (6,8%) in RALL-2009 and 19/170 (11,2%) in RALL-2016). But the total number of allo-HSCTs was somewhat higher in RALL-2016: 21/305 (6,9%) in RALL-2009 and 25/186 (13,5%), p=0,02).
Results
Two trials look quite similar in main characteristics, though pts in RALL-2016 were older. Both trials have demonstrated identical stable results with optimistic long-term outcome (Pic.1. A,B). High early death rate (~10%) remains an obstacle that decreases CR rate and overall survival especially in Regional centers. MRD-positivity discriminated BCP-ALL patients at day+70 (Pic.1C) and T-ALL patients at day +133 (Pic.1D) to a poor prognostic group.
Conclusion
It was shown that disregard the absence of high-dose consolidations with high-dose methotrexate/ARA-C and low numbers of allo-HSCT in 1CR, both trials had demonstrated stable, high and reproducible results regarding overall and disease-free survival. In our setting MRD-positivity at day+70 for BCP-ALL and at day 133+ for T-ALL identifies patients that need different approach aimed to early MRD treatment with targeting drugs followed by allo-HSCT.
Keyword(s): Acute lymphoblastic leukemia
Abstract: EP360
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Since 2009 RALL-study group has conducted two clinical multicenter trials on the treatment of adult patients with Ph-negative ALL. The majority of adult ALL studies are highly intensive with substantial numbers of allogeneic HSCT in 1CR and tailor their treatment according to MRD levels after the induction phase. RALL studies were based on the non-intensive, but non-interruptive approach without high dose consolidations and without many allo-HSCTs but with autologous HSCT for T-cell ALL as late consolidation. Pregnant women were included in both trials. MRD measurement was carried out only in the second RALL-2016 study.
Aims
to present the comparison of long-term results of both studies and evaluate the impact of MRD measurement in RALL-2016.
Methods
From Jan, 2009, till Dec, 2016, 30 centers had enrolled 330 and since Dec 2016 till Jan 2021, 10 centers included 207 acute Ph-negative lymphoblastic leukemia patients. The main trials characteristics are presented in Table 1. There were no difference between two trials in numbers of autologous HSCTs for T-ALL (42/125 (33%) pts in RALL-2009 and 23/85 (27%) in RALL-2016); no difference in numbers of allo-HSCTs in 1CR (19/281 (6,8%) in RALL-2009 and 19/170 (11,2%) in RALL-2016). But the total number of allo-HSCTs was somewhat higher in RALL-2016: 21/305 (6,9%) in RALL-2009 and 25/186 (13,5%), p=0,02).
Results
Two trials look quite similar in main characteristics, though pts in RALL-2016 were older. Both trials have demonstrated identical stable results with optimistic long-term outcome (Pic.1. A,B). High early death rate (~10%) remains an obstacle that decreases CR rate and overall survival especially in Regional centers. MRD-positivity discriminated BCP-ALL patients at day+70 (Pic.1C) and T-ALL patients at day +133 (Pic.1D) to a poor prognostic group.

Conclusion
It was shown that disregard the absence of high-dose consolidations with high-dose methotrexate/ARA-C and low numbers of allo-HSCT in 1CR, both trials had demonstrated stable, high and reproducible results regarding overall and disease-free survival. In our setting MRD-positivity at day+70 for BCP-ALL and at day 133+ for T-ALL identifies patients that need different approach aimed to early MRD treatment with targeting drugs followed by allo-HSCT.
Keyword(s): Acute lymphoblastic leukemia


