
Contributions
Abstract: EP357
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
The outcome of relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) is dismal. The only evaluable effective, though quite toxic, therapeutic option is Nelarabine combinations followed by allogeneic HSCT (allo-HSCT). Recent preclinical studies and few case reports suggested that inhibition of antiapoptotic protein BCL-2 could be effective in T-cell ALL and this effect could be potentiated by hypomethylating agents.
Aims
We have conducted a small pilot study aiming to evaluate the efficacy of venetoclax with decitabine in the treatment of MRD persistent and relapsed/refractory T-cell ALL patients (pts) included in the RALL-2016 study (NCT01193933).
Methods
Since February 2019 till January 2021 seven T-ALL pts were included: 5 m, 2 f, median age 32 (range 19-39) yy. Two pts (ETP-TI-ALL and T-III ALL) had persisting MRD at day +133/+190 of the study, 3 had overt relapses (1 ETP-ALL, 1 T-I/II ALL, 1 T-II ALL), two – refractory (1 TII-ALL, 1 T/B ALL). Three pts (42%) had a complex karyotype, one (14%) – deletion in chromosome 17p [del(17p)]. All pts were monitored for MRD by MFC. Decitabine 20 mg/m2 was infused i.v. for 5 days, Venetoclax was used 400 mg by mouth daily in 28 day cycles. Two pts (29%) had second relapse after previous Nelarabine exposure followed by allo-HSCT.
Results
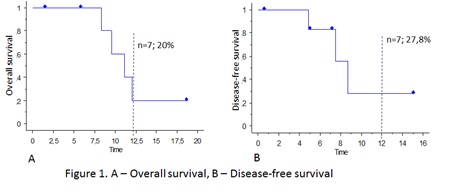
All of the 5 pts with relapse/primary refractory Т-cell ALL achieved complete remission, 4 (80%) of them reached MRD-negativity. Both pts with MRD persistence achieved MRD negativity. The median time to remission was 0.9 month (range 0.7 – 2.1 months). Six pts (86%) underwent subsequent allo-HSCT, for one of them it was the second allo-HSCT. One patient is awaiting for allo-HSCT. The median follow-up since the start of venetoclax was 9,5 months (range 1,6 – 18,8). Three pts are alive (43%), 4 died (57%), and the 1-year OS in the whole cohort was 20% (Figure 1a). In total, 3 of the 7 pts had relapsed, and the median DFS was 7.3 months (0,6 – 15,14). The 1-year DFS was 27,8% (Figure 2b).
Conclusion
Though very small our study has demonstrated high efficacy (six out of seven deep responses) with low toxicity in the extremely poor prognostic group of pts. Though the responses are short and relapses occurred even after allo-HSCT. It could be supposed that the pts need after allo-transplant treatment with the same Ven+Dac combination.
Keyword(s): Relapsed acute lymphoblastic leukemia, T-ALL
Abstract: EP357
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
The outcome of relapsed/refractory T-cell acute lymphoblastic leukemia (ALL) is dismal. The only evaluable effective, though quite toxic, therapeutic option is Nelarabine combinations followed by allogeneic HSCT (allo-HSCT). Recent preclinical studies and few case reports suggested that inhibition of antiapoptotic protein BCL-2 could be effective in T-cell ALL and this effect could be potentiated by hypomethylating agents.
Aims
We have conducted a small pilot study aiming to evaluate the efficacy of venetoclax with decitabine in the treatment of MRD persistent and relapsed/refractory T-cell ALL patients (pts) included in the RALL-2016 study (NCT01193933).
Methods
Since February 2019 till January 2021 seven T-ALL pts were included: 5 m, 2 f, median age 32 (range 19-39) yy. Two pts (ETP-TI-ALL and T-III ALL) had persisting MRD at day +133/+190 of the study, 3 had overt relapses (1 ETP-ALL, 1 T-I/II ALL, 1 T-II ALL), two – refractory (1 TII-ALL, 1 T/B ALL). Three pts (42%) had a complex karyotype, one (14%) – deletion in chromosome 17p [del(17p)]. All pts were monitored for MRD by MFC. Decitabine 20 mg/m2 was infused i.v. for 5 days, Venetoclax was used 400 mg by mouth daily in 28 day cycles. Two pts (29%) had second relapse after previous Nelarabine exposure followed by allo-HSCT.
Results
All of the 5 pts with relapse/primary refractory Т-cell ALL achieved complete remission, 4 (80%) of them reached MRD-negativity. Both pts with MRD persistence achieved MRD negativity. The median time to remission was 0.9 month (range 0.7 – 2.1 months). Six pts (86%) underwent subsequent allo-HSCT, for one of them it was the second allo-HSCT. One patient is awaiting for allo-HSCT. The median follow-up since the start of venetoclax was 9,5 months (range 1,6 – 18,8). Three pts are alive (43%), 4 died (57%), and the 1-year OS in the whole cohort was 20% (Figure 1a). In total, 3 of the 7 pts had relapsed, and the median DFS was 7.3 months (0,6 – 15,14). The 1-year DFS was 27,8% (Figure 2b).

Conclusion
Though very small our study has demonstrated high efficacy (six out of seven deep responses) with low toxicity in the extremely poor prognostic group of pts. Though the responses are short and relapses occurred even after allo-HSCT. It could be supposed that the pts need after allo-transplant treatment with the same Ven+Dac combination.
Keyword(s): Relapsed acute lymphoblastic leukemia, T-ALL


