
Contributions
Abstract: EP353
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
InO is a CD22-directed antibody-drug conjugate indicated for treatment of relapsed/refractory (R/R) ALL. InO has been associated with hepatotoxicity and hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), particularly post-HSCT.
Aims
To assess toxicity in patients (pts) with ALL who received InO prior to HSCT, utilizing registry data from the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
CIBMTR patient data are being collected for a 5-year period after US approval of InO (Aug 2017 – Aug 2022). Data from US pts age ≥18 y treated with InO who proceeded to allogeneic HSCT were included. Using interim data at 3 y, we evaluated post-HSCT outcomes, including clinical status, overall survival, transplant-related (non-relapse) mortality (NRM), relapse, death after relapse (time from HSCT to death after the first 28 d from any cause with prior relapse/progression post-HSCT), and investigator-defined adverse events, including hepatic VOD/SOS. All statistical analyses are descriptive.
Results
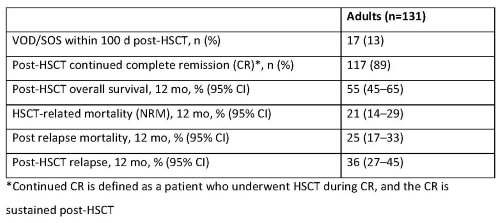
Data accrued from 18 Aug 2017 to 17 Aug 2020 for 131 adult pts (median age 40 y) who proceeded to first allogeneic HSCT: 31% in first complete remission (CR1), 46% in CR2, 13% in ≥CR3, 5% in 1st relapse, 2% in ≥3rd relapse, and 3% in primary induction failure. A majority (70%) had transplants from peripheral blood stem cells, and 47% involved an HLA-identical sibling or other related donor. Nearly half received myeloablative conditioning regimens. Before HSCT, 36% of pts received 1 cycle of InO, 46% had 2 cycles, and 17% had ≥3 cycles. Half (48%) received InO as a single agent. Median time from last dose of InO to HSCT was 2.0 mos (range: 0.4–26.2). At the time of data lock (11 Nov 2020), post-transplant data were available for 131 pts. Outcomes for these pts are shown in the Table. Among a subgroup of adults with active R/R ALL (n=91) at the time of HSCT (median of 4 lines of prior therapy), VOD/SOS incidence within 100 d of HSCT was 18%.
Conclusion
Incidence of VOD/SOS after first HSCT in InO-treated pts with R/R ALL in this study was similar to the 18-19% reported in pooled analyses of 2 clinical trials among InO-treated pts with R/R ALL (Marks et al, Biol Blood Marrow Transplant 2019) and in the INOVATE study (Kantarjian et al, Lancet Haematol 2017). The NRM at 1 y of 21% (23% R/R ALL) is lower than the NRM at 1 y of 38% reported in the pooled analyses of R/R ALL InO recipients.
Keyword(s): ALL, B cell acute lymphoblastic leukemia, HSCT, Veno-occlusive disease
Abstract: EP353
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
InO is a CD22-directed antibody-drug conjugate indicated for treatment of relapsed/refractory (R/R) ALL. InO has been associated with hepatotoxicity and hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), particularly post-HSCT.
Aims
To assess toxicity in patients (pts) with ALL who received InO prior to HSCT, utilizing registry data from the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
CIBMTR patient data are being collected for a 5-year period after US approval of InO (Aug 2017 – Aug 2022). Data from US pts age ≥18 y treated with InO who proceeded to allogeneic HSCT were included. Using interim data at 3 y, we evaluated post-HSCT outcomes, including clinical status, overall survival, transplant-related (non-relapse) mortality (NRM), relapse, death after relapse (time from HSCT to death after the first 28 d from any cause with prior relapse/progression post-HSCT), and investigator-defined adverse events, including hepatic VOD/SOS. All statistical analyses are descriptive.
Results
Data accrued from 18 Aug 2017 to 17 Aug 2020 for 131 adult pts (median age 40 y) who proceeded to first allogeneic HSCT: 31% in first complete remission (CR1), 46% in CR2, 13% in ≥CR3, 5% in 1st relapse, 2% in ≥3rd relapse, and 3% in primary induction failure. A majority (70%) had transplants from peripheral blood stem cells, and 47% involved an HLA-identical sibling or other related donor. Nearly half received myeloablative conditioning regimens. Before HSCT, 36% of pts received 1 cycle of InO, 46% had 2 cycles, and 17% had ≥3 cycles. Half (48%) received InO as a single agent. Median time from last dose of InO to HSCT was 2.0 mos (range: 0.4–26.2). At the time of data lock (11 Nov 2020), post-transplant data were available for 131 pts. Outcomes for these pts are shown in the Table. Among a subgroup of adults with active R/R ALL (n=91) at the time of HSCT (median of 4 lines of prior therapy), VOD/SOS incidence within 100 d of HSCT was 18%.

Conclusion
Incidence of VOD/SOS after first HSCT in InO-treated pts with R/R ALL in this study was similar to the 18-19% reported in pooled analyses of 2 clinical trials among InO-treated pts with R/R ALL (Marks et al, Biol Blood Marrow Transplant 2019) and in the INOVATE study (Kantarjian et al, Lancet Haematol 2017). The NRM at 1 y of 21% (23% R/R ALL) is lower than the NRM at 1 y of 38% reported in the pooled analyses of R/R ALL InO recipients.
Keyword(s): ALL, B cell acute lymphoblastic leukemia, HSCT, Veno-occlusive disease


