
Contributions
Abstract: EP352
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Despite a high complete remission (CR) rate is obtained with frontline therapy, relapses are a frequent event in T-ALL, with limited salvage options to date. Studies on outcome of patients (pts) with R/R T-ALL treated with MRD-oriented therapies are scarce.
Aims
We analyzed the outcome of pts with R/R T-ALL included in two successive MRD-oriented trials (ALL-AR-03 and ALL-HR-11) from the Spanish PETHEMA Group.
Methods
Retrospective study of R/R T-ALL adults diagnosed between 2003 and 2019 and included in the ALL-AR-03 (NCT00853008) and ALL-HR-11 (NCT01540812) protocols. The clinical characteristics at baseline and at relapse, the salvage therapies and outcomes (CR and OS) were analyzed, and a study of prognostic factors for response to rescue regimens and OS was performed.
Results
Seventy-four pts were identified (ALL-HR03 [n=36], ALL HR-11 [n=38], refractory [n=5], and relapsed [n=69]). Median age (range) at diagnosis was 31 (16-58) yrs., 57 were males (77%), with CNS involvement in 9 (12%), mediastinal mass 32 (43%), PB blast count 30.6 x109/L (0-284), early T-cell precursor (ETP) 17 (25%), pre-T 11 (16%), cortical 27 (40%), mature 13 (19%), T-unspecified 5. Post-induction-1 MRD level ≥0.1% was found in 13/56 pts (23%), and ≥0.01%: in 20/53 (38%). Fourteen pts (19%) received 2nd induction therapy (resistant disease after induction-1 [n=9], MRD≥0.1% after induction-1 [n=5]). Allogeneic-HSCT was performed in CR1 to 11/69 pts (16%). Interval CR1-relapse: 9.4 (0.1-36.7) months.
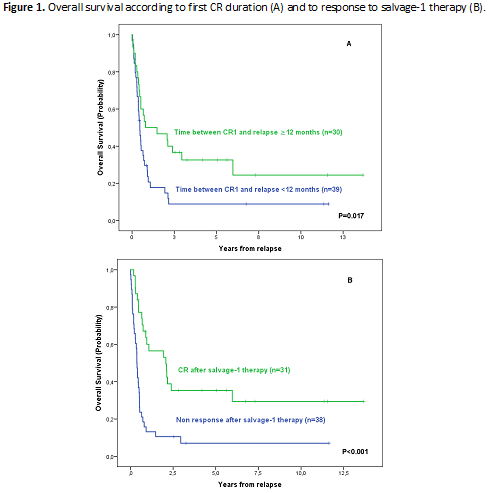
Relapse was located in BM (n=29, 42%), BM+extramedullary (n=20, 29%) and extramedullary (n=20, 29%). CNS involvement at relapse was found in 23 pts (33%, isolated in 11 cases). Median number of salvage lineages was 2 (range: 1-5). The most frequent salvage-1 schedules were FLAG-Ida (n=41, 56%), HyperCVAD (n=9, 12%) and nelarabine (n=6, 8%) (other schedules: 18 pts). Second CR was attained in 33/74 pts (44%), being higher for patients treated with FLAG-Ida vs. the remaining regimens (23/41 (56%) vs 10/33 (30%), p=0.026). The CR rate and outcome were not significantly different on comparison of ETP vs. the remaining T-ALL subtypes. Good morphologic and/or MRD response after Induction-1 in first line therapy and salvage treatment with FLAG-IDA were independently associated with CR after salvage therapy (OR: 5.077 [95%CI 1.275-20.218] and 2.879 [95%CI 1.052-7.876], respectively). Allogeneic-HSCT was performed to 35 pts (29 in CR2). Sixty pts died (progression: 34, toxicity of rescue regimens: 15, TRM: 11). Median OS (95%CI) was 6.2 (3.9-8.6) months, with a 5yr OS probability of 18% (9%>27%). By multivariable analysis, only the interval between CR1 and relapse >12 months and CR after salvage-1 therapy emerged as favorable prognostic factors for OS (HR 2.071, 95%CI: 1.185-3.622 and HR 3.227, 95% CI: 1.859-5.780, respectively) (Figure 1 A-B).
Conclusion
This study shows poor outcome of adults with R/R T-ALL, with CR to first salvage therapy of 44% and median OS of 6 months. Good cytological or MRD response to first line therapy and FLAG-Ida rescue therapy were associated with better response to salvage-1 treatment, whereas late relapse and response to salvage-1 regimen were associated with better OS. This study highlights the unmet need for novel effective therapies for T-ALL.
Supported in part by grant 2017 SGR288 (GRC) Generalitat de Catalunya and “La Caixa” Foundation and ISCIII (PI19/01828).
Keyword(s): Outcome, Relapse, Salvage therapy, T-ALL
Abstract: EP352
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Despite a high complete remission (CR) rate is obtained with frontline therapy, relapses are a frequent event in T-ALL, with limited salvage options to date. Studies on outcome of patients (pts) with R/R T-ALL treated with MRD-oriented therapies are scarce.
Aims
We analyzed the outcome of pts with R/R T-ALL included in two successive MRD-oriented trials (ALL-AR-03 and ALL-HR-11) from the Spanish PETHEMA Group.
Methods
Retrospective study of R/R T-ALL adults diagnosed between 2003 and 2019 and included in the ALL-AR-03 (NCT00853008) and ALL-HR-11 (NCT01540812) protocols. The clinical characteristics at baseline and at relapse, the salvage therapies and outcomes (CR and OS) were analyzed, and a study of prognostic factors for response to rescue regimens and OS was performed.
Results
Seventy-four pts were identified (ALL-HR03 [n=36], ALL HR-11 [n=38], refractory [n=5], and relapsed [n=69]). Median age (range) at diagnosis was 31 (16-58) yrs., 57 were males (77%), with CNS involvement in 9 (12%), mediastinal mass 32 (43%), PB blast count 30.6 x109/L (0-284), early T-cell precursor (ETP) 17 (25%), pre-T 11 (16%), cortical 27 (40%), mature 13 (19%), T-unspecified 5. Post-induction-1 MRD level ≥0.1% was found in 13/56 pts (23%), and ≥0.01%: in 20/53 (38%). Fourteen pts (19%) received 2nd induction therapy (resistant disease after induction-1 [n=9], MRD≥0.1% after induction-1 [n=5]). Allogeneic-HSCT was performed in CR1 to 11/69 pts (16%). Interval CR1-relapse: 9.4 (0.1-36.7) months.
Relapse was located in BM (n=29, 42%), BM+extramedullary (n=20, 29%) and extramedullary (n=20, 29%). CNS involvement at relapse was found in 23 pts (33%, isolated in 11 cases). Median number of salvage lineages was 2 (range: 1-5). The most frequent salvage-1 schedules were FLAG-Ida (n=41, 56%), HyperCVAD (n=9, 12%) and nelarabine (n=6, 8%) (other schedules: 18 pts). Second CR was attained in 33/74 pts (44%), being higher for patients treated with FLAG-Ida vs. the remaining regimens (23/41 (56%) vs 10/33 (30%), p=0.026). The CR rate and outcome were not significantly different on comparison of ETP vs. the remaining T-ALL subtypes. Good morphologic and/or MRD response after Induction-1 in first line therapy and salvage treatment with FLAG-IDA were independently associated with CR after salvage therapy (OR: 5.077 [95%CI 1.275-20.218] and 2.879 [95%CI 1.052-7.876], respectively). Allogeneic-HSCT was performed to 35 pts (29 in CR2). Sixty pts died (progression: 34, toxicity of rescue regimens: 15, TRM: 11). Median OS (95%CI) was 6.2 (3.9-8.6) months, with a 5yr OS probability of 18% (9%>27%). By multivariable analysis, only the interval between CR1 and relapse >12 months and CR after salvage-1 therapy emerged as favorable prognostic factors for OS (HR 2.071, 95%CI: 1.185-3.622 and HR 3.227, 95% CI: 1.859-5.780, respectively) (Figure 1 A-B).

Conclusion
This study shows poor outcome of adults with R/R T-ALL, with CR to first salvage therapy of 44% and median OS of 6 months. Good cytological or MRD response to first line therapy and FLAG-Ida rescue therapy were associated with better response to salvage-1 treatment, whereas late relapse and response to salvage-1 regimen were associated with better OS. This study highlights the unmet need for novel effective therapies for T-ALL.
Supported in part by grant 2017 SGR288 (GRC) Generalitat de Catalunya and “La Caixa” Foundation and ISCIII (PI19/01828).
Keyword(s): Outcome, Relapse, Salvage therapy, T-ALL


