
Contributions
Abstract: EP351
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Philadelphia-like (Ph-like) acute lymphoblastic leukemia (ALL) is a high-risk disease associated with high rates of persistent minimal residual disease (MRD+) and relapse rate when treated with conventional chemotherapy. Several novel therapies such as blinatumomab (BLINA), inotuzumab (INO), CAR T cell therapy (CAR), and venetoclax-based regimens have shown promising activity in treatment of relapsed/refractory (r/r) B-cell ALL regardless of prior chemo-refractoriness or high-risk genetics; but the impact of Ph-like genotype on response to novel therapies in r/r ALL remains largely unknown.
Aims
Here, we retrospectively analyzed CR/CRi rates in adults with Ph-like ALL who were treated at City of Hope with novel agents from 2013 to 2021.
Methods
To identify the Ph-like status, we utilized our Anchored Multiplex PCR™-based multi-gene NGS assay designed to detect known and novel fusions, and elevated gene expression levels using a proprietary algorithm in ALL.
Results
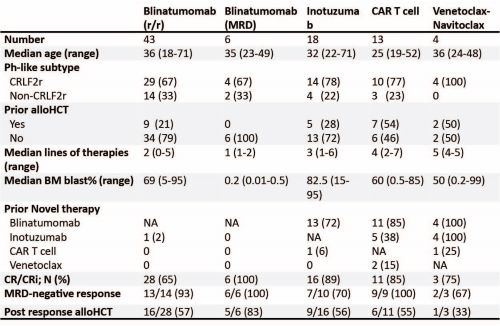
We identified 67 adult patients with Ph-like ALL treated with 84 novel therapies. The median age was 33 years (range: 18-71) and the majority were males (n=45; 67%) and Hispanics (n=57; 85%). CRLF2r was detected in 49 (73%) patients. BLINA was administered to 43 (64%) and 6 (9%) patients for overt relapsed/refractory (r/r) or persistent MRD+ ALL, respectively. Eighteen patients (27%) received INO, 13 (19%) received CD19CAR and 4 (6%) were treated with venetoclax-navitoclax. Median number of prior lines of therapies were 2, 3, and 4 for patients treated with BLINA, INO, and CAR, respectively. Only one patient who was treated with BLINA received prior INO, while 72% and 6% of patients treated with INO had BLINA and CAR before, and 85% and 38% of patients treated with CAR had BLINA and INO before, respectively.
Complete remission/complete remission with incomplete hematologic recovery (CR/Cri) rates were 64%, 89% and 85% in patients treated with BLINA, INO and CAR, respectively. MRD- rate was 93%, 70%, and 100% in responders who received BLINA, INO, and Car, respectively. Once in CR, 57%, 56% and 55% of responders to BLINA, INO and CAR underwent allogeneic hematopoietic cell transplantation (HCT) consolidation. 12-month relapse-free survival for responders from the time of BLINA, INO and CAR response were 67%, 41% and 75% (p=0.54), respectively. All 6 patients with Ph-like ALL treated with BLINA in MRD setting achieved MRD, of whom 5 received consolidation with HCT; and 3 out of 4 patients treated with venetoclax-navitoclax achieved CR/CRi and only one received HCT afterward.
Conclusion
In conclusion, treatment of r/r Ph-like ALL with novel therapies resulted in excellent CR/CRi and MRD- response rates and successfully transitioned over half of patients to HCT. Response rates to INO and CAR were high despite most patients in these treatment groups had prior treatment with BLINA. Integrating these novel therapies in the frontline setting could improve outcomes of Ph-like ALL and overcome their refractoriness to conventional chemotherapy.
Keyword(s): Acute lymphoblastic leukemia, Immunotherapy
Abstract: EP351
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Philadelphia-like (Ph-like) acute lymphoblastic leukemia (ALL) is a high-risk disease associated with high rates of persistent minimal residual disease (MRD+) and relapse rate when treated with conventional chemotherapy. Several novel therapies such as blinatumomab (BLINA), inotuzumab (INO), CAR T cell therapy (CAR), and venetoclax-based regimens have shown promising activity in treatment of relapsed/refractory (r/r) B-cell ALL regardless of prior chemo-refractoriness or high-risk genetics; but the impact of Ph-like genotype on response to novel therapies in r/r ALL remains largely unknown.
Aims
Here, we retrospectively analyzed CR/CRi rates in adults with Ph-like ALL who were treated at City of Hope with novel agents from 2013 to 2021.
Methods
To identify the Ph-like status, we utilized our Anchored Multiplex PCR™-based multi-gene NGS assay designed to detect known and novel fusions, and elevated gene expression levels using a proprietary algorithm in ALL.
Results
We identified 67 adult patients with Ph-like ALL treated with 84 novel therapies. The median age was 33 years (range: 18-71) and the majority were males (n=45; 67%) and Hispanics (n=57; 85%). CRLF2r was detected in 49 (73%) patients. BLINA was administered to 43 (64%) and 6 (9%) patients for overt relapsed/refractory (r/r) or persistent MRD+ ALL, respectively. Eighteen patients (27%) received INO, 13 (19%) received CD19CAR and 4 (6%) were treated with venetoclax-navitoclax. Median number of prior lines of therapies were 2, 3, and 4 for patients treated with BLINA, INO, and CAR, respectively. Only one patient who was treated with BLINA received prior INO, while 72% and 6% of patients treated with INO had BLINA and CAR before, and 85% and 38% of patients treated with CAR had BLINA and INO before, respectively.
Complete remission/complete remission with incomplete hematologic recovery (CR/Cri) rates were 64%, 89% and 85% in patients treated with BLINA, INO and CAR, respectively. MRD- rate was 93%, 70%, and 100% in responders who received BLINA, INO, and Car, respectively. Once in CR, 57%, 56% and 55% of responders to BLINA, INO and CAR underwent allogeneic hematopoietic cell transplantation (HCT) consolidation. 12-month relapse-free survival for responders from the time of BLINA, INO and CAR response were 67%, 41% and 75% (p=0.54), respectively. All 6 patients with Ph-like ALL treated with BLINA in MRD setting achieved MRD, of whom 5 received consolidation with HCT; and 3 out of 4 patients treated with venetoclax-navitoclax achieved CR/CRi and only one received HCT afterward.

Conclusion
In conclusion, treatment of r/r Ph-like ALL with novel therapies resulted in excellent CR/CRi and MRD- response rates and successfully transitioned over half of patients to HCT. Response rates to INO and CAR were high despite most patients in these treatment groups had prior treatment with BLINA. Integrating these novel therapies in the frontline setting could improve outcomes of Ph-like ALL and overcome their refractoriness to conventional chemotherapy.
Keyword(s): Acute lymphoblastic leukemia, Immunotherapy


