
Contributions
Abstract: EP345
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Blinatumomab (BLINA) administration induces high response rates for treatment of relapsed/refractory (R/R) and persistent minimal residual disease (MRD+) acute lymphoblastic leukemia (ALL). A few reports have addressed the unique pattern of BLINA-failure during ALL therapy, manifesting either as extramedullary (EM) progression/relapse (P/R) or loss of CD19 antigen expression. However, EM P/R ALL phenomena during BLINA therapy remains largely underrecognized and is not fully understood.
Aims
We perform this study to identify the prevalence and factors influencing EM R/P in ALL following BLINA therapy.
Methods
We retrospectively reviewed outcomes of adults with B-cell ALL who were treated with BLINA at City of Hope between 2013 and 2021, and analyzed potential factors impacting response and EM P/R following BLINA therapy.
Results
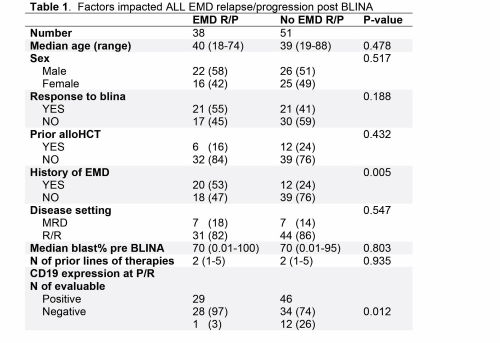
We identified 132 patients treated with BLINA for either R/R (n= 103; 78%) or MRD+ (n= 29; 22%) B cell ALL. Median age at the time of BLINA treatment was 39 years (range:18-88). Higher response rate to BLINA was associated with treatment in the MRD+ ALL setting (p=0.008), lower bone marrow (BM) blast burden in R/R setting (p=0.029), no history of prior EM disease (p=0.001), and fewer lines of pre-BLINA therapies (p=0.048). In multivariable analysis, only lower pre-BLINA BM blast burden (p=0.049) and the lack of EM disease history (p=0.019) remained significantly associated with higher response to BLINA. BLINA failure (defined as initial progression in non-responders or post BLINA relapse in responders) was noted in 89 patients. At the time of BLINA failure, 38 (43%) patients P/R EM, including 24 patients as isolated EM disease without BM involvement. 17 (36%) patients of those were refractory to BLINA and 21 (50%) responders to BLINA who relapsed subsequently progressed EM. History of EM involvement (53% vs. 24%, p= 0.005) and P/R with CD19+ ALL (97% vs. 74%, p= 0.012) were associated with higher risk for EM P/R at the time of BLINA failure. Factors did not impact EM P/R include; response to BLINA, pre-BLINA stem cell transplant, disease setting (MRD vs. R/R), or pre-BLINA BM blast burden. EM P/R involving the central nervous system (CNS) occurred in 15 (39%) patients, and more frequently encountered in responders at the time of relapse (n= 12; 57%) compared to refractory patients at the time of progression (n= 3; 18%).
Conclusion
In conclusion, EM P/R is common during BLINA therapy in ALL, especially in responders; since half of them relapsed EM (+/-BM). History of EM disease during ALL course predicted inferior response to BLINA and higher risk for P/R EM. CNS P/R is frequently observed at the time of BLINA failure, and the majority of extramedullary P/R cases retained CD19 expression. Therefore, BLINA therapy should be cautiously used in patients with history of EM disease and patients should be closely monitored for EM progression during treatment.
Keyword(s): Acute lymphoblastic leukemia, Immunotherapy
Abstract: EP345
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Clinical
Background
Blinatumomab (BLINA) administration induces high response rates for treatment of relapsed/refractory (R/R) and persistent minimal residual disease (MRD+) acute lymphoblastic leukemia (ALL). A few reports have addressed the unique pattern of BLINA-failure during ALL therapy, manifesting either as extramedullary (EM) progression/relapse (P/R) or loss of CD19 antigen expression. However, EM P/R ALL phenomena during BLINA therapy remains largely underrecognized and is not fully understood.
Aims
We perform this study to identify the prevalence and factors influencing EM R/P in ALL following BLINA therapy.
Methods
We retrospectively reviewed outcomes of adults with B-cell ALL who were treated with BLINA at City of Hope between 2013 and 2021, and analyzed potential factors impacting response and EM P/R following BLINA therapy.
Results
We identified 132 patients treated with BLINA for either R/R (n= 103; 78%) or MRD+ (n= 29; 22%) B cell ALL. Median age at the time of BLINA treatment was 39 years (range:18-88). Higher response rate to BLINA was associated with treatment in the MRD+ ALL setting (p=0.008), lower bone marrow (BM) blast burden in R/R setting (p=0.029), no history of prior EM disease (p=0.001), and fewer lines of pre-BLINA therapies (p=0.048). In multivariable analysis, only lower pre-BLINA BM blast burden (p=0.049) and the lack of EM disease history (p=0.019) remained significantly associated with higher response to BLINA. BLINA failure (defined as initial progression in non-responders or post BLINA relapse in responders) was noted in 89 patients. At the time of BLINA failure, 38 (43%) patients P/R EM, including 24 patients as isolated EM disease without BM involvement. 17 (36%) patients of those were refractory to BLINA and 21 (50%) responders to BLINA who relapsed subsequently progressed EM. History of EM involvement (53% vs. 24%, p= 0.005) and P/R with CD19+ ALL (97% vs. 74%, p= 0.012) were associated with higher risk for EM P/R at the time of BLINA failure. Factors did not impact EM P/R include; response to BLINA, pre-BLINA stem cell transplant, disease setting (MRD vs. R/R), or pre-BLINA BM blast burden. EM P/R involving the central nervous system (CNS) occurred in 15 (39%) patients, and more frequently encountered in responders at the time of relapse (n= 12; 57%) compared to refractory patients at the time of progression (n= 3; 18%).

Conclusion
In conclusion, EM P/R is common during BLINA therapy in ALL, especially in responders; since half of them relapsed EM (+/-BM). History of EM disease during ALL course predicted inferior response to BLINA and higher risk for P/R EM. CNS P/R is frequently observed at the time of BLINA failure, and the majority of extramedullary P/R cases retained CD19 expression. Therefore, BLINA therapy should be cautiously used in patients with history of EM disease and patients should be closely monitored for EM progression during treatment.
Keyword(s): Acute lymphoblastic leukemia, Immunotherapy


