Contributions
Abstract: EP330
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Erdheim-Chester disease (ECD) belongs to a group of rare hematological diseases and is characterized histologically by proliferation of mature histiocytes on a background of inflammatory stroma. The disease is systemic, with clinical manifestations ranging from focal indolent bone lesions to a multi-systemic, life-threatening disease. We have recently reported an aberrant expression of microRNAs (miRNAs) in ECD patients (Weissman et al, Cancers, 2020). NanoString analysis revealed downregulation of 95% of differentially expressed miRNAs in ECD patients compared to healthy controls. Subsequent bioinformatics analysis suggested that lower expression of certain miRNAs in ECD results in upregulation of target genes that participate in cell survival signaling and inflammation. The most prominent and significant change detected between ECD and healthy controls was downregulation of miR-15a-5p. The miR-15/16 cluster represents the most frequently deregulated miRNAs reported in hematological malignancies, and has been associated with disease progression, prognosis, and drug resistance.
Aims
To study the biological role of miR-15a-5p in ECD pathogenesis.
Methods
Global miRNA expression was analyzed in plasma samples of untreated ECD patients (n=34) and healthy controls (n=15) using the NanoString nCounter miRNA assay. As several lines of evidence have suggested that ECD may originate from a myeloid progenitor, we used the human myeloid cell lines KG-1a and OCI-AML3 as a model to study the biological effects of miR-15a-5p. Cytokine-dependent murine pro-B Ba/F3 lymphoid cells that stably expressed the MIGII-BRAFV600E vector were also used. The effects of aberrantly expressed miRNAs on potential molecular targets was analyzed using quantitative real-time polymerase chain reaction (qRT-PCR), luciferase assay, enzyme-linked immunosorbent assay (ELISA), Western analysis, proliferation assay, cell-cycle analysis and Annexin-propium iodide staining.
Results
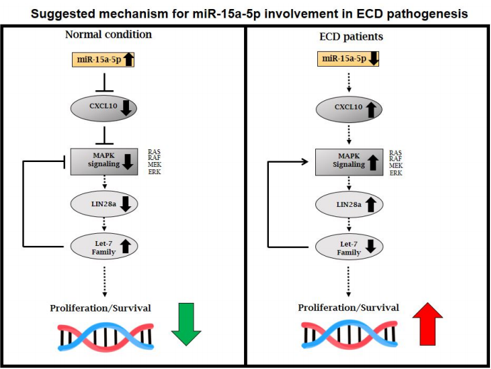
Bioinformatics target predictor (TargetScan) identified a conserved target site for miRNA-15a-5p in the 3’ UTR of the chemokine ligand 10 (CXCL10). A luciferase assay confirmed that CXCL10 is a target gene regulated by miRNA-15a-5p. Low levels of miR-15a-5p in ECD patients were associated with upregulation of CXCL10 in patients plasma samples. Increased expression of miR-15a-5p, by transfection of miR-15a-5p mimic, resulted in CXCL10 downregulation, followed by downregulation of the oncogene LIN28a and p-ERK signaling, leading to upregulation of the tumor suppressor let-7 family miRNAs. Furthermore, overexpression of miR-15a-5p in cell lines and in a primary culture, derived from an ECD patient, inhibited cell growth and induced apoptosis by decreased levels of Bcl-2 and Bcl-xl. Finally, analysis of sequential samples from 7 ECD patients treated with MAPK inhibitors (vemurafenib/cobimetinib) for 4 months resulted in upregulation of miR-15a-5p and downregulation of CXCL10.
Conclusion
Our findings strongly suggest that miR-15a-5p acts as a tumor suppressor in ECD patients through the CXCL10-ERK-LIN28a-let7 axis, highlighting an additional layer of post-transcriptional regulation in this disease. Upregulation of miR-15a-5p in ECD patients may have a potential therapeutic role.
Keyword(s): Apoptosis, Cell cycle, Inflammation, MAP kinase
Abstract: EP330
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Erdheim-Chester disease (ECD) belongs to a group of rare hematological diseases and is characterized histologically by proliferation of mature histiocytes on a background of inflammatory stroma. The disease is systemic, with clinical manifestations ranging from focal indolent bone lesions to a multi-systemic, life-threatening disease. We have recently reported an aberrant expression of microRNAs (miRNAs) in ECD patients (Weissman et al, Cancers, 2020). NanoString analysis revealed downregulation of 95% of differentially expressed miRNAs in ECD patients compared to healthy controls. Subsequent bioinformatics analysis suggested that lower expression of certain miRNAs in ECD results in upregulation of target genes that participate in cell survival signaling and inflammation. The most prominent and significant change detected between ECD and healthy controls was downregulation of miR-15a-5p. The miR-15/16 cluster represents the most frequently deregulated miRNAs reported in hematological malignancies, and has been associated with disease progression, prognosis, and drug resistance.
Aims
To study the biological role of miR-15a-5p in ECD pathogenesis.
Methods
Global miRNA expression was analyzed in plasma samples of untreated ECD patients (n=34) and healthy controls (n=15) using the NanoString nCounter miRNA assay. As several lines of evidence have suggested that ECD may originate from a myeloid progenitor, we used the human myeloid cell lines KG-1a and OCI-AML3 as a model to study the biological effects of miR-15a-5p. Cytokine-dependent murine pro-B Ba/F3 lymphoid cells that stably expressed the MIGII-BRAFV600E vector were also used. The effects of aberrantly expressed miRNAs on potential molecular targets was analyzed using quantitative real-time polymerase chain reaction (qRT-PCR), luciferase assay, enzyme-linked immunosorbent assay (ELISA), Western analysis, proliferation assay, cell-cycle analysis and Annexin-propium iodide staining.
Results
Bioinformatics target predictor (TargetScan) identified a conserved target site for miRNA-15a-5p in the 3’ UTR of the chemokine ligand 10 (CXCL10). A luciferase assay confirmed that CXCL10 is a target gene regulated by miRNA-15a-5p. Low levels of miR-15a-5p in ECD patients were associated with upregulation of CXCL10 in patients plasma samples. Increased expression of miR-15a-5p, by transfection of miR-15a-5p mimic, resulted in CXCL10 downregulation, followed by downregulation of the oncogene LIN28a and p-ERK signaling, leading to upregulation of the tumor suppressor let-7 family miRNAs. Furthermore, overexpression of miR-15a-5p in cell lines and in a primary culture, derived from an ECD patient, inhibited cell growth and induced apoptosis by decreased levels of Bcl-2 and Bcl-xl. Finally, analysis of sequential samples from 7 ECD patients treated with MAPK inhibitors (vemurafenib/cobimetinib) for 4 months resulted in upregulation of miR-15a-5p and downregulation of CXCL10.
Conclusion
Our findings strongly suggest that miR-15a-5p acts as a tumor suppressor in ECD patients through the CXCL10-ERK-LIN28a-let7 axis, highlighting an additional layer of post-transcriptional regulation in this disease. Upregulation of miR-15a-5p in ECD patients may have a potential therapeutic role.
Keyword(s): Apoptosis, Cell cycle, Inflammation, MAP kinase