
Contributions
Abstract: EP329
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Translocation t(1;19)(q23;p13) encoding the TCF3-PBX1 fusion gene is considered an intermediate-risk B-cell precursor acute lymphoblastic leukemia (BCP ALL) subtype. In adult BCP ALL there is a debate regarding the impact of this alteration. While German, English and American study groups have shown no prognostic significance, French and Italian groups have reported poor outcomes.
Aims
To analyze the frequency and clinical impact of t(1;19) in a series of adult BCP ALL patients (pts) treated with protocols from the PETHEMA Group.
Methods
A review of 513 adult BCP ALL pts (15 to 60 years) diagnosed between 2003 and 2017 and treated with MRD-oriented protocols of the PETHEMA Group was done. G-banding and FISH were performed on BM samples. Measurable residual disease (MRD) was centrally assessed by multi-parametric flow cytometry. Main outcome measures were complete response (CR), overall survival (OS) and cumulative incidence of relapse (CIR), assessed by competing risk analysis.
Results
A total of 26 pts with t(1;19)/TCF3-PBX1 were identified, representing 5% of all BCP ALL. 9/23 (39%) cases showed isolated t(1;19) while 14/23 (61%) had additional chromosomal aberrations (ACA) (in 3 cases the TCF3-PBX1 fusion was detected by FISH in the context of a normal karyotype). Pts with t(1;19) were more likely to be female (73% vs. 45%, p=0.006) and to have a pre-B phenotype (63% vs. 17%, p<0.001). Although TCF3-PBX1 pts showed higher WBC count than the remaining patients, the difference was not statistically significant (median 16.2 [0-199.5] vs. 12 [0.23-638] x109/L, p=0.144).
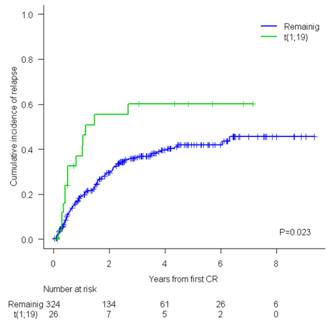
CR was achieved in 100% of t(1;19) vs. 90%, in the remaining BCP ALL pts (p=0.151). The proportion of pts with end induction MRD level <0.01% was similar (70% vs. 61%, p=0.417). No differences in OS were observed (5y-OS t(1:19) 41% [19%>63%] vs. 44% [38%>50%], p=0.562). Pts with t(1;19) experienced more deaths due to disease progression (77% vs. 59%) and less deaths due to treatment-related toxicity (15% vs. 38%). No differences were observed in OS among pts with isolated t(1;19) and those with t(1;19) with ACA (5y-OS 59% [23%>95%] vs. 33% [1%>65%], p=0.645). A significantly higher CIR was observed for patients with t(1;19) compared with the remaining BCP ALL pts (5y-CIR 60% [36%>77%] vs. 42% [35%>48%], p=0.023)(Figure). The 5y-CIR of patients with isolated t(1;19) showed a trend to be lower to that of t(1;19) with ACA (28% [3%>63%] vs. 71% [36%>89%], p=0.119). All outcome parameters were also calculated for patients with balanced t(1;19) or unbalanced der(19)t(1;19) without statistical differences between them.
Conclusion
Although showing favorable initial treatment response, pts with t(1;19) experience a higher rate of relapse (especially those with ACA to t(1;19)) than the remaining BCP ALL pts, without differences in OS. A deeper genetic analysis may identify markers of poor outcome enabling a more precise risk stratification of t(1;19) pts.
Keyword(s): Acute lymphoblastic leukemia, Adult, Outcome
Abstract: EP329
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Translocation t(1;19)(q23;p13) encoding the TCF3-PBX1 fusion gene is considered an intermediate-risk B-cell precursor acute lymphoblastic leukemia (BCP ALL) subtype. In adult BCP ALL there is a debate regarding the impact of this alteration. While German, English and American study groups have shown no prognostic significance, French and Italian groups have reported poor outcomes.
Aims
To analyze the frequency and clinical impact of t(1;19) in a series of adult BCP ALL patients (pts) treated with protocols from the PETHEMA Group.
Methods
A review of 513 adult BCP ALL pts (15 to 60 years) diagnosed between 2003 and 2017 and treated with MRD-oriented protocols of the PETHEMA Group was done. G-banding and FISH were performed on BM samples. Measurable residual disease (MRD) was centrally assessed by multi-parametric flow cytometry. Main outcome measures were complete response (CR), overall survival (OS) and cumulative incidence of relapse (CIR), assessed by competing risk analysis.
Results
A total of 26 pts with t(1;19)/TCF3-PBX1 were identified, representing 5% of all BCP ALL. 9/23 (39%) cases showed isolated t(1;19) while 14/23 (61%) had additional chromosomal aberrations (ACA) (in 3 cases the TCF3-PBX1 fusion was detected by FISH in the context of a normal karyotype). Pts with t(1;19) were more likely to be female (73% vs. 45%, p=0.006) and to have a pre-B phenotype (63% vs. 17%, p<0.001). Although TCF3-PBX1 pts showed higher WBC count than the remaining patients, the difference was not statistically significant (median 16.2 [0-199.5] vs. 12 [0.23-638] x109/L, p=0.144).
CR was achieved in 100% of t(1;19) vs. 90%, in the remaining BCP ALL pts (p=0.151). The proportion of pts with end induction MRD level <0.01% was similar (70% vs. 61%, p=0.417). No differences in OS were observed (5y-OS t(1:19) 41% [19%>63%] vs. 44% [38%>50%], p=0.562). Pts with t(1;19) experienced more deaths due to disease progression (77% vs. 59%) and less deaths due to treatment-related toxicity (15% vs. 38%). No differences were observed in OS among pts with isolated t(1;19) and those with t(1;19) with ACA (5y-OS 59% [23%>95%] vs. 33% [1%>65%], p=0.645). A significantly higher CIR was observed for patients with t(1;19) compared with the remaining BCP ALL pts (5y-CIR 60% [36%>77%] vs. 42% [35%>48%], p=0.023)(Figure). The 5y-CIR of patients with isolated t(1;19) showed a trend to be lower to that of t(1;19) with ACA (28% [3%>63%] vs. 71% [36%>89%], p=0.119). All outcome parameters were also calculated for patients with balanced t(1;19) or unbalanced der(19)t(1;19) without statistical differences between them.

Conclusion
Although showing favorable initial treatment response, pts with t(1;19) experience a higher rate of relapse (especially those with ACA to t(1;19)) than the remaining BCP ALL pts, without differences in OS. A deeper genetic analysis may identify markers of poor outcome enabling a more precise risk stratification of t(1;19) pts.
Keyword(s): Acute lymphoblastic leukemia, Adult, Outcome


