
Contributions
Abstract: EP321
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
15% of pediatric and 40% of adult T-cell Acute Lymphoblastic Leukemia (T-ALL) patients fail conventional therapy highlighting the need for novel therapeutic strategies based on the genomic alterations of individuals.
Aims
We interrogated genomic alterations in Australian T-ALL patients for patterns of mutation and druggable targets. A subset of samples were used to establish patient-derived xenograft (PDX) models to evaluate novel therapies.
Methods
T-ALL patients’ samples underwent transcriptomic analyses (148 total samples including diagnosis, refractory and relapse timepoints from 137 patients of all age groups). mRNA sequencing (mRNAseq) identified gene fusions and structural variants and assessed differential gene expression (n=120). Fusion calling relied on FusionCatcher, SOAPfuse, JAFFA; variant calling utilized GATK HaplotypeCaller. Possible germline alterations and common SNPs were filtered prior to manual curation. DNA copy number variations (CNVs) were detected via MLPA (MRC Holland, P202/P335/P383; n=80). Establishment of PDX models from patient material (bone marrow, peripheral blood) is ongoing.
Results
Genomic fusion genes were identified in 58/120 samples (48%) by mRNAseq; the most common was STIL-TAL1 (n=9). Increased expression of LCK and/or LAT (encoded proteins are involved in T-cell receptor (TCR) signal transduction) was observed in patients harbouring STIL-TAL1 indicating TCR signaling may be perturbed in this sub-group. Other common gene fusions were MLLT10-DDX3X (n=5) and KMT2A-MLLT4/AFDN (n=4). We also observed SET-NUP214, KMT2A-MLLT1, PICALM-MLLT10, NUP214-ABL1, several fusions involving TCR subunits as well as novel fusions involving KMT2A, NOTCH1, SUZ12, CTCF, ZEB2.
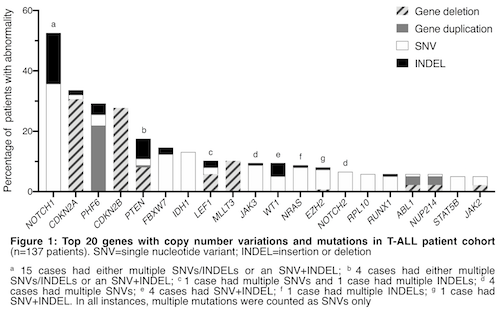
Nonsynonymous mutations were identified in 106/120 patients (88%) with mRNAseq data. Broadly, mutated genes encode proteins in the following categories: oncoproteins (NRAS, KRAS); tumour suppressors (TP53, PTEN); epigenetic regulators (DNMT3A, EZH2, IDH1/2, PHF6); regulators of NOTCH signalling (NOTCH1/2, FBXW7); transcription factors and regulators (ETV6, IKZF1, LEF1, RUNX1, WT1); kinase and cytokine signal regulators (JAK1, JAK2, JAK3, TYK2). Clinically relevant INDELS were identified in 67/120 patients (56%) including alterations to: CDKN2A, FBXW7, IL7R, JAK3, LEF1, NOTCH1, PHF6, PTEN, WT1.
The most common copy number alterations identified were CDKN2A/B deletions (37/80 patients, 46%), PHF6 duplication (30/80 patients, 38%) and MLLT3 deletion (14/80 patients, 18%). In patients with homozygous CDKN2A/B deletions and additional CNVs, PHF6 duplication (n=9) and MYB duplication (n=4) were mutually exclusive. MLLT3 deletion always co-occurred with CDKN2A/B deletions (19/19 patients with MLLT3 deletion), but was rarely observed with PTEN deletion (1/11 patients with PTEN deletion). Patients with either CDKN2A/B deletions or PHF6 duplications frequently harbored NOTCH1 abnormalities: 24/36 patients (67%) and 15/26 patients (58%), respectively.
PDX primagrafts investigated the engraftment latency and peripheral organ infiltration. Primagrafts established from patients harboring gene fusions typically engrafted at a slower rate (n=4, 70-93 days) than primagrafts from patients harboring CDKN2A/B deletions (n=2, 32-53 days).
Conclusion
In our cohort the majority of cases harbor rearrangements, structural variations and duplication/deletion of genes associated with malignant transformation. We identified several co-occurring lesions as well as mutually exclusive genomic abnormalities. Secondary PDX models investigating targeted treatment strategies are ongoing.
Keyword(s): Clinical data, Mouse model, T cell acute lymphoblastic leukemia, T-ALL
Abstract: EP321
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
15% of pediatric and 40% of adult T-cell Acute Lymphoblastic Leukemia (T-ALL) patients fail conventional therapy highlighting the need for novel therapeutic strategies based on the genomic alterations of individuals.
Aims
We interrogated genomic alterations in Australian T-ALL patients for patterns of mutation and druggable targets. A subset of samples were used to establish patient-derived xenograft (PDX) models to evaluate novel therapies.
Methods
T-ALL patients’ samples underwent transcriptomic analyses (148 total samples including diagnosis, refractory and relapse timepoints from 137 patients of all age groups). mRNA sequencing (mRNAseq) identified gene fusions and structural variants and assessed differential gene expression (n=120). Fusion calling relied on FusionCatcher, SOAPfuse, JAFFA; variant calling utilized GATK HaplotypeCaller. Possible germline alterations and common SNPs were filtered prior to manual curation. DNA copy number variations (CNVs) were detected via MLPA (MRC Holland, P202/P335/P383; n=80). Establishment of PDX models from patient material (bone marrow, peripheral blood) is ongoing.
Results
Genomic fusion genes were identified in 58/120 samples (48%) by mRNAseq; the most common was STIL-TAL1 (n=9). Increased expression of LCK and/or LAT (encoded proteins are involved in T-cell receptor (TCR) signal transduction) was observed in patients harbouring STIL-TAL1 indicating TCR signaling may be perturbed in this sub-group. Other common gene fusions were MLLT10-DDX3X (n=5) and KMT2A-MLLT4/AFDN (n=4). We also observed SET-NUP214, KMT2A-MLLT1, PICALM-MLLT10, NUP214-ABL1, several fusions involving TCR subunits as well as novel fusions involving KMT2A, NOTCH1, SUZ12, CTCF, ZEB2.
Nonsynonymous mutations were identified in 106/120 patients (88%) with mRNAseq data. Broadly, mutated genes encode proteins in the following categories: oncoproteins (NRAS, KRAS); tumour suppressors (TP53, PTEN); epigenetic regulators (DNMT3A, EZH2, IDH1/2, PHF6); regulators of NOTCH signalling (NOTCH1/2, FBXW7); transcription factors and regulators (ETV6, IKZF1, LEF1, RUNX1, WT1); kinase and cytokine signal regulators (JAK1, JAK2, JAK3, TYK2). Clinically relevant INDELS were identified in 67/120 patients (56%) including alterations to: CDKN2A, FBXW7, IL7R, JAK3, LEF1, NOTCH1, PHF6, PTEN, WT1.
The most common copy number alterations identified were CDKN2A/B deletions (37/80 patients, 46%), PHF6 duplication (30/80 patients, 38%) and MLLT3 deletion (14/80 patients, 18%). In patients with homozygous CDKN2A/B deletions and additional CNVs, PHF6 duplication (n=9) and MYB duplication (n=4) were mutually exclusive. MLLT3 deletion always co-occurred with CDKN2A/B deletions (19/19 patients with MLLT3 deletion), but was rarely observed with PTEN deletion (1/11 patients with PTEN deletion). Patients with either CDKN2A/B deletions or PHF6 duplications frequently harbored NOTCH1 abnormalities: 24/36 patients (67%) and 15/26 patients (58%), respectively.
PDX primagrafts investigated the engraftment latency and peripheral organ infiltration. Primagrafts established from patients harboring gene fusions typically engrafted at a slower rate (n=4, 70-93 days) than primagrafts from patients harboring CDKN2A/B deletions (n=2, 32-53 days).

Conclusion
In our cohort the majority of cases harbor rearrangements, structural variations and duplication/deletion of genes associated with malignant transformation. We identified several co-occurring lesions as well as mutually exclusive genomic abnormalities. Secondary PDX models investigating targeted treatment strategies are ongoing.
Keyword(s): Clinical data, Mouse model, T cell acute lymphoblastic leukemia, T-ALL


