
Contributions
Abstract: EP317
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Acquired genetic changes are essential diagnostic and prognostic markers in acute lymphoblastic leukaemia (ALL) used in the risk stratification of patients for treatment.
Chromosomal translocations result in either the formation of a fusion protein, or, in the case of translocations involving the Immunoglobulin heavy chain locus (IGH-t), they result in the activation of proto-oncogenes driven by strong regulatory elements.
IGH-t are observed in 5-10% of B-cell precursor ALL (BCP-ALL) and are associated with a poor prognosis in adults 1. The IGH locus is highly promiscuous, rearranging with many different chromosomal partners. These observations led us to question the consequences of such rearrangements and their impact on gene expression signatures. Do all IGH-t lead to deregulation of the same signalling pathways or can we subdivide patients according to the function of the partner gene involved?
Aims
We hypothesise that the transcriptomic and clinical characteristics of IGH-t ALL are determined by the translocation partner gene. The aims of this study are (1) to characterise the transcriptome of IGH-t ALL; (2) to identify any potential druggable pathways to improve treatment options.
Methods
RNA sequencing data from 270 BCP-ALL cases (195 cases from Lund University (EGAS00001001795)3 and 75 in-house cases), including 52 IGH-t cases, were processed for cluster analysis, with LASSO feature selection using label in Li et al.4, followed by hierarchical cluster analysis. Gene set enrichment analysis (GSEA) was performed to identify enrichment in drug-related gene sets. The results were validated in vitro using patient derived xenograft cells (PDX). Drug experiments were conducted using an in-house high-throughput drug screening system.
Results
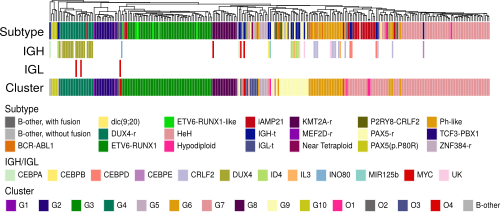
Cluster analysis revealed that IGH-DUX4 plus IGH-CRLF2 and IGH-IL3 clustered together with DUX4-rearranged (G4) and BCR-ABL1(-like) (G6) cases, respectively (Fig. 1). One novel observation was that IGH-ID4 cases, which were reported to associate with PAX5 deletions5, clustered with PAX5-rearranged (PAX5-r) and P2RY8-CRLF2 (G9) cases (Fig. 1). GSEA indicated that IGH-DUX4 ALL may have reduced sensitivity to asparaginase, characterised by higher expression of ribosome-related genes and GATA3. Asparaginase treatment of PDX samples revealed a higher IC50 for IGH-DUX4 (n=1) compared to non-IGH-DUX4 PDX (n=2) (logIC50 : 0.4791 vs -2.896). Addition of dexamethasone (1 nM), a GATA3 phorsphorylation inhibitor, enhanced the inhibition of IGH-DUX4 PDX by asparaginase (logIC50: -2.761), suggesting potential synergism (Average ZIP score: 5.377).
Conclusion
IGH-t ALL comprises a heterogeneous subtype in which further subtyping may provide insight into novel therapeutic avenues. Patients with IGH-DUX4 and IGH-CRLF2 clustered with DUX4-r and BCR-ABL1-like cases, as expected.We report a novel observation that IGH-ID4 patients cluster with PAX5-r ALL. Although IGH-DUX4 ALL has been shown to confer a good prognosis, our study suggests these patients may have reduced sensitivity to asparaginase, a drug used in induction phase chemotherapy. Further investigation of their response to induction phase chemotherapy is required to understand its clinical implication.
References
1. Russell et al. J Clin Oncol. 2014 May 10;32(14):1453-62
2. Roberts et al. N Engl J Med. 2014 Sep 11; 371(11): 1005–1015.
3. Lilljebjörn et al. Nat Commun. 2016 Jun 6;7:11790.
4. Li et al. Proc Natl Acad Sci USA. 2018 Dec 11;115(50):E11711-E11720.
5. Russell et al. Blood. 2008 Jan 1;111(1):387-91.
Keyword(s): B cell acute lymphoblastic leukemia, Drug resistance, IgH rearrangment, IgH translocation
Abstract: EP317
Type: E-Poster Presentation
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Acquired genetic changes are essential diagnostic and prognostic markers in acute lymphoblastic leukaemia (ALL) used in the risk stratification of patients for treatment.
Chromosomal translocations result in either the formation of a fusion protein, or, in the case of translocations involving the Immunoglobulin heavy chain locus (IGH-t), they result in the activation of proto-oncogenes driven by strong regulatory elements.
IGH-t are observed in 5-10% of B-cell precursor ALL (BCP-ALL) and are associated with a poor prognosis in adults 1. The IGH locus is highly promiscuous, rearranging with many different chromosomal partners. These observations led us to question the consequences of such rearrangements and their impact on gene expression signatures. Do all IGH-t lead to deregulation of the same signalling pathways or can we subdivide patients according to the function of the partner gene involved?
Aims
We hypothesise that the transcriptomic and clinical characteristics of IGH-t ALL are determined by the translocation partner gene. The aims of this study are (1) to characterise the transcriptome of IGH-t ALL; (2) to identify any potential druggable pathways to improve treatment options.
Methods
RNA sequencing data from 270 BCP-ALL cases (195 cases from Lund University (EGAS00001001795)3 and 75 in-house cases), including 52 IGH-t cases, were processed for cluster analysis, with LASSO feature selection using label in Li et al.4, followed by hierarchical cluster analysis. Gene set enrichment analysis (GSEA) was performed to identify enrichment in drug-related gene sets. The results were validated in vitro using patient derived xenograft cells (PDX). Drug experiments were conducted using an in-house high-throughput drug screening system.
Results
Cluster analysis revealed that IGH-DUX4 plus IGH-CRLF2 and IGH-IL3 clustered together with DUX4-rearranged (G4) and BCR-ABL1(-like) (G6) cases, respectively (Fig. 1). One novel observation was that IGH-ID4 cases, which were reported to associate with PAX5 deletions5, clustered with PAX5-rearranged (PAX5-r) and P2RY8-CRLF2 (G9) cases (Fig. 1). GSEA indicated that IGH-DUX4 ALL may have reduced sensitivity to asparaginase, characterised by higher expression of ribosome-related genes and GATA3. Asparaginase treatment of PDX samples revealed a higher IC50 for IGH-DUX4 (n=1) compared to non-IGH-DUX4 PDX (n=2) (logIC50 : 0.4791 vs -2.896). Addition of dexamethasone (1 nM), a GATA3 phorsphorylation inhibitor, enhanced the inhibition of IGH-DUX4 PDX by asparaginase (logIC50: -2.761), suggesting potential synergism (Average ZIP score: 5.377).

Conclusion
IGH-t ALL comprises a heterogeneous subtype in which further subtyping may provide insight into novel therapeutic avenues. Patients with IGH-DUX4 and IGH-CRLF2 clustered with DUX4-r and BCR-ABL1-like cases, as expected.We report a novel observation that IGH-ID4 patients cluster with PAX5-r ALL. Although IGH-DUX4 ALL has been shown to confer a good prognosis, our study suggests these patients may have reduced sensitivity to asparaginase, a drug used in induction phase chemotherapy. Further investigation of their response to induction phase chemotherapy is required to understand its clinical implication.
References
1. Russell et al. J Clin Oncol. 2014 May 10;32(14):1453-62
2. Roberts et al. N Engl J Med. 2014 Sep 11; 371(11): 1005–1015.
3. Lilljebjörn et al. Nat Commun. 2016 Jun 6;7:11790.
4. Li et al. Proc Natl Acad Sci USA. 2018 Dec 11;115(50):E11711-E11720.
5. Russell et al. Blood. 2008 Jan 1;111(1):387-91.
Keyword(s): B cell acute lymphoblastic leukemia, Drug resistance, IgH rearrangment, IgH translocation


