
Contributions
Abstract: EP1341
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Patients with haematological malignancies more commonly present with thrombocytopenia than any other cohort. Early identification of the cause of thrombocytopenia is important to provide timely intervention to decrease the risk of life-threatening bleeding. Sysmex XN analysers allow for the measurement of immature platelet fraction (IPF%), differentiating mature from immature platelets. IPF% has the ability to distinguish between the causes of thrombocytopenia, differentiating decreased platelet production from increased platelet destruction. IPF% also has the ability to predict platelet recovery, reducing unnecessary platelet transfusions in patients where the platelet count would recover without intervention.
Aims
The aim of this project was to determine the efficacy in IPF% in determining the cause of thrombocytopenia and to determine the ability of IPF% to predict platelet recovery in thrombocytopenic patients in UHW.
Methods
87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). Patients with normal FBC parameters (n=100) were included to establish a reference range for IPF%, represented using the 2.5th and 97.5th percentile. Comparison of IPF% amongst each group was carried out using Mann-Whitney U test. Reproducibility of IPF% was assessed by coefficient of variation (CV%) analysis. 14 patients were followed daily through platelet count and IPF% to determine the ability of IPF% to predict platelet recovery. ROC curve analysis was performed to determine the diagnostic cut off point of IPF% in differentiating between ITP and aplastic anaemia. ROC curve analysis was also used to determine the optimal value of IPF% for the prediction of platelet recovery.
Results
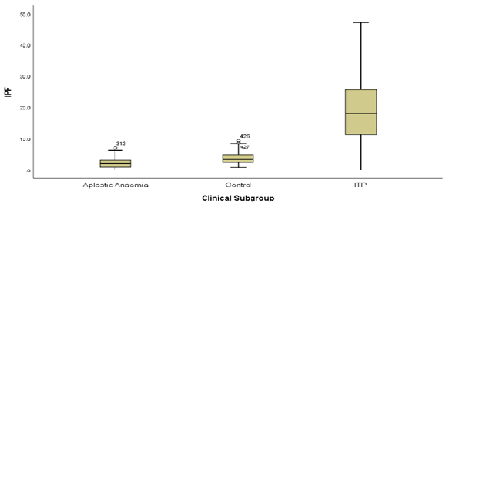
IPF% was assessed in patients with ITP, aplastic anaemia and a control group. A statistically significant different in IPF% between ITP and AA patients (p<0.05) and the control group (p<0.05) was found. ROC curve analysis determined a cut of point of 6.8% for differentiating ITP from AA with a sensitivity of 87% and specificity of 92.9%. To determine the sensitivity and specificity of IPF% in predicting imminent platelet recovery, ROC curve analysis was used. The increase in IPF% for ROC curve analysis was defined as the increase in IPF% from platelet nadir to the peak IPF% prior to platelet recovery. An increase of 2.1% was found to have a sensitivity and specificity of 92% and 96% respectively in predicting platelet recovery within 2 days of increase in IPF%.
Conclusion
Extensive analysis of the data obtained for this study has shown the benefits to IPF% as a clinical biomarker. Implementation of IPF% as a routine parameter would be beneficial to clinicians in UHW. As a non-invasive biomarker, IPF% would allow clinicians to gain an insight into the cause of thrombocytopenia allowing for prompt decisions regarding treatment strategies without the need for invasive procedures. IPF% as a predictive biomarker would also allow clinicians to defer platelet transfusion if platelet recovery is imminent, avoiding unwarranted platelet transfusions.
Keyword(s): Idiopathic thombocytopenic purpura (ITP), Prediction, Thrombocytopenia
Abstract: EP1341
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Patients with haematological malignancies more commonly present with thrombocytopenia than any other cohort. Early identification of the cause of thrombocytopenia is important to provide timely intervention to decrease the risk of life-threatening bleeding. Sysmex XN analysers allow for the measurement of immature platelet fraction (IPF%), differentiating mature from immature platelets. IPF% has the ability to distinguish between the causes of thrombocytopenia, differentiating decreased platelet production from increased platelet destruction. IPF% also has the ability to predict platelet recovery, reducing unnecessary platelet transfusions in patients where the platelet count would recover without intervention.
Aims
The aim of this project was to determine the efficacy in IPF% in determining the cause of thrombocytopenia and to determine the ability of IPF% to predict platelet recovery in thrombocytopenic patients in UHW.
Methods
87 thrombocytopenic patients were included over a 13-month period. Patients were divided into two groups; decreased platelet production (n=53) and increased platelet destruction (n=34). Patients with normal FBC parameters (n=100) were included to establish a reference range for IPF%, represented using the 2.5th and 97.5th percentile. Comparison of IPF% amongst each group was carried out using Mann-Whitney U test. Reproducibility of IPF% was assessed by coefficient of variation (CV%) analysis. 14 patients were followed daily through platelet count and IPF% to determine the ability of IPF% to predict platelet recovery. ROC curve analysis was performed to determine the diagnostic cut off point of IPF% in differentiating between ITP and aplastic anaemia. ROC curve analysis was also used to determine the optimal value of IPF% for the prediction of platelet recovery.
Results
IPF% was assessed in patients with ITP, aplastic anaemia and a control group. A statistically significant different in IPF% between ITP and AA patients (p<0.05) and the control group (p<0.05) was found. ROC curve analysis determined a cut of point of 6.8% for differentiating ITP from AA with a sensitivity of 87% and specificity of 92.9%. To determine the sensitivity and specificity of IPF% in predicting imminent platelet recovery, ROC curve analysis was used. The increase in IPF% for ROC curve analysis was defined as the increase in IPF% from platelet nadir to the peak IPF% prior to platelet recovery. An increase of 2.1% was found to have a sensitivity and specificity of 92% and 96% respectively in predicting platelet recovery within 2 days of increase in IPF%.

Conclusion
Extensive analysis of the data obtained for this study has shown the benefits to IPF% as a clinical biomarker. Implementation of IPF% as a routine parameter would be beneficial to clinicians in UHW. As a non-invasive biomarker, IPF% would allow clinicians to gain an insight into the cause of thrombocytopenia allowing for prompt decisions regarding treatment strategies without the need for invasive procedures. IPF% as a predictive biomarker would also allow clinicians to defer platelet transfusion if platelet recovery is imminent, avoiding unwarranted platelet transfusions.
Keyword(s): Idiopathic thombocytopenic purpura (ITP), Prediction, Thrombocytopenia


