
Contributions
Abstract: EP1338
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Autoimmune hemolytic anemia (AIHA) is characterized by the presence of anti-red blood cell (RBC) autoantibodies with active hemolysis. Several studies have described a higher thrombotic risk compared to healthy subjects, possibly related to a combination of autoimmune features and hemolysis. However, the relationship between presence of anti-erythrocyte autoantibody and risk of thrombosis has not yet been explored.
Aims
The aim of this study is to identify whether the elevated thrombotic risk in AIHA could be associated with anti-RBC autoantibody presence regardless of the existence of active hemolysis.
Methods
We conducted a retrospective research in a single-center cohort selecting patients with an identified anti-RBC autoantibody and then evaluating the presence of hemolysis and the incidence of thrombotic events.
Laboratory methods to identify autoantibodies included direct antiglobulin test (polyspecific and monospecific anti-IgG and C3d, Menarini reagents) and elution tests (acid glycine elution with ELU-KIT Immucor or 56ºC elution) were also undertaken. Measured parameters for hemolysis were indirect bilirubin, LDH and reticulocytes.
Patients with AIHA were recorded from 2009, and those without active hemolysis were registered during part of this follow-up period. We also assessed associated diseases and features of the autoantibody. Prothrombotic characteristics (elderly, active cancer, previous VTE, known thrombophilic condition, recent trauma/surgery, infection/rheumatologic disorder and hormonal treatment) were registered in order to identify bias. Reduced mobility could not be evaluated due to lack of data.
Results
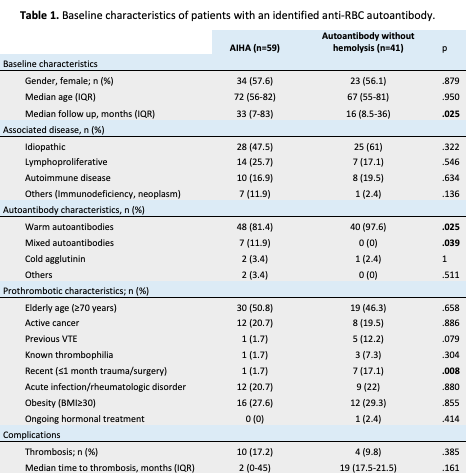
105 patients with an anti-RBC autoantibody were identified, 5 were excluded due to lack of information. Among these 100 patients, 59 showed active hemolysis (AIHA). There was no difference in gender, age or associated diseases (idiopathic, lymphoproliferative, autoimmune disorders or others) between both groups. Patients without hemolysis showed more frequently warm autoantibody features (p=0.025) and less commonly mixed characteristics (p=0.039). Median follow up in AIHA patients was higher (33 vs 16 months; p=0.025). Prothrombotic factors were comparable among both groups, except for recent trauma/surgery (p=0.008), which was more frequent in patients without hemolytic features. We found a thrombosis incidence in AIHA patients of 17.2%; which is consistent with previous studies and comparable to those without hemolytic features (17.2% vs 9.8%; p=0.39. We dismissed the presence of confounding factors (trauma/surgery, warm or mixed autoantibodies) through stratified analysis, finding no statistical significance (p>0.14) on the incidence of thrombosis between AIHA and patients without hemolysis.
Median time to thrombosis from autoantibody identification was shorter in AIHA patients (2 vs 19 months; p=0.16) as 4 of them presented venous thromboembolism (VTE) at diagnosis. VTE in AIHA and patients without hemolysis were pulmonary embolism (5 vs 2); deep vein thrombosis (3 vs 2); and portal vein thrombosis (2 vs 0).
Conclusion
Thrombotic events among patients with anti-RBC autoantibody were similar, regardless of the presence of active hemolysis (AIHA). Despite AIHA features and even in the absence of hemolysis, patients with anti-RBC autoantibodies may benefit from a closer follow up in order to prevent thrombosis. Further studies are needed to clear the significance of these autoantibodies in the pathogeny of AIHA related thrombosis.
Keyword(s): Autoantibody, Autoimmune hemolytic anemia (AIHA), Hemolysis, Thrombosis
Abstract: EP1338
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Autoimmune hemolytic anemia (AIHA) is characterized by the presence of anti-red blood cell (RBC) autoantibodies with active hemolysis. Several studies have described a higher thrombotic risk compared to healthy subjects, possibly related to a combination of autoimmune features and hemolysis. However, the relationship between presence of anti-erythrocyte autoantibody and risk of thrombosis has not yet been explored.
Aims
The aim of this study is to identify whether the elevated thrombotic risk in AIHA could be associated with anti-RBC autoantibody presence regardless of the existence of active hemolysis.
Methods
We conducted a retrospective research in a single-center cohort selecting patients with an identified anti-RBC autoantibody and then evaluating the presence of hemolysis and the incidence of thrombotic events.
Laboratory methods to identify autoantibodies included direct antiglobulin test (polyspecific and monospecific anti-IgG and C3d, Menarini reagents) and elution tests (acid glycine elution with ELU-KIT Immucor or 56ºC elution) were also undertaken. Measured parameters for hemolysis were indirect bilirubin, LDH and reticulocytes.
Patients with AIHA were recorded from 2009, and those without active hemolysis were registered during part of this follow-up period. We also assessed associated diseases and features of the autoantibody. Prothrombotic characteristics (elderly, active cancer, previous VTE, known thrombophilic condition, recent trauma/surgery, infection/rheumatologic disorder and hormonal treatment) were registered in order to identify bias. Reduced mobility could not be evaluated due to lack of data.
Results
105 patients with an anti-RBC autoantibody were identified, 5 were excluded due to lack of information. Among these 100 patients, 59 showed active hemolysis (AIHA). There was no difference in gender, age or associated diseases (idiopathic, lymphoproliferative, autoimmune disorders or others) between both groups. Patients without hemolysis showed more frequently warm autoantibody features (p=0.025) and less commonly mixed characteristics (p=0.039). Median follow up in AIHA patients was higher (33 vs 16 months; p=0.025). Prothrombotic factors were comparable among both groups, except for recent trauma/surgery (p=0.008), which was more frequent in patients without hemolytic features. We found a thrombosis incidence in AIHA patients of 17.2%; which is consistent with previous studies and comparable to those without hemolytic features (17.2% vs 9.8%; p=0.39. We dismissed the presence of confounding factors (trauma/surgery, warm or mixed autoantibodies) through stratified analysis, finding no statistical significance (p>0.14) on the incidence of thrombosis between AIHA and patients without hemolysis.
Median time to thrombosis from autoantibody identification was shorter in AIHA patients (2 vs 19 months; p=0.16) as 4 of them presented venous thromboembolism (VTE) at diagnosis. VTE in AIHA and patients without hemolysis were pulmonary embolism (5 vs 2); deep vein thrombosis (3 vs 2); and portal vein thrombosis (2 vs 0).

Conclusion
Thrombotic events among patients with anti-RBC autoantibody were similar, regardless of the presence of active hemolysis (AIHA). Despite AIHA features and even in the absence of hemolysis, patients with anti-RBC autoantibodies may benefit from a closer follow up in order to prevent thrombosis. Further studies are needed to clear the significance of these autoantibodies in the pathogeny of AIHA related thrombosis.
Keyword(s): Autoantibody, Autoimmune hemolytic anemia (AIHA), Hemolysis, Thrombosis


