
Contributions
Abstract: EP1337
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Results from the International PNH Registry have shown that eculizumab reduced the number of transfusions by 50% in patients (pts) with PNH with transfusion history. Ravulizumab, the new standard of care for PNH, was non-inferior to eculizumab in 2 large clinical trials; 77% and 87% of pts treated with ravulizumab achieved transfusion avoidance at 1 year. Collecting real world data on the proportion of pts with PNH requiring transfusions whilst on ravulizumab, or after switching from eculizumab to ravulizumab (E-R switch) is important and valuable. Electronic medical records (EMR) and claims databases provide valuable real-world insight.
Aims
Determine proportion of pts with PNH receiving transfusions before and after initiating treatment with eculizumab, ravulizumab or E-R switch by using large-scale US EMR and claims databases.
Methods
TriNetX is a global health research network that integrates data from a variety of sources to create longitudinal real-world data, which can be sourced from EMR or claims. KOMODO Health is a comprehensive claims database in the US. The study population was identified using records with ICD-10 codes for PNH. Pts were grouped into three cohorts: received eculizumab but never ravulizumab (eculizumab-only), received ravulizumab but never eculizumab (ravulizumab-only), and an E-R switch. The TriNetX platform was used to access data up to 10/Feb/21 from two TriNetX networks: the US EMR and US Claims networks. Analyses for these databases were conducted at 6-month intervals: 6-months (mos) to 1 day pre-treatment, 0-6-mos post-treatment and continued at 6-month intervals thereafter until 18-24 mos in ravulizumab and E-R switch cohorts, and 42–48 mos in the eculizumab cohort. From the KOMODO Health database, claims data were sourced from 01/Jan/15 to 31/Dec/19. Analyses were conducted at 3-month intervals: 3 mos to 0-days pre-treatment, day of treatment (index date) to 3 mos post-treatment and at 3-month intervals until 6 mos for the ravulizumab and E-R switch cohorts, and 24 mos for the eculizumab cohort. Proportion of pts receiving transfusions at each interval was assessed.
Results
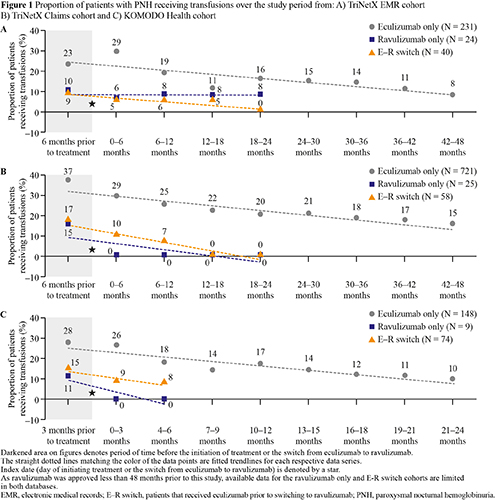
A total of 295, 804 and 231 pts with PNH that met the analysis criteria were identified from TriNetX EMR, TriNetX Claims and KOMODO, respectively (Figure 1). Proportion of pts receiving transfusions prior to index date in the eculizumab-only cohort was higher in all 3 databases. In the eculizumab-only cohort, the proportion of pts receiving transfusions decreased from 23–37% pre-treatment to 8–15% at the end of the study period. In the ravulizumab-only cohort, proportion of pts receiving transfusions decreased from 10–15% pre-treatment to 0% in the TriNetX Claims and KOMODO cohorts, and 8% in the TriNetX EMR cohort at the end of the study period. In the E-R switch cohort, proportion of pts receiving transfusions decreased from 9–17% pre-switch, to 0% in TriNetX Claims and TriNetX EMR cohorts and 8% in the KOMODO cohort at the end of study period after switching to ravulizumab. A limitation of this study was the small ravulizumab sample size.
Conclusion
Results of this real-world study demonstrate that the proportion of pts with PNH requiring blood transfusions was substantially reduced with eculizumab or ravulizumab monotherapy or after the E-R switch, with similar trends noted between treatment groups. This real-world data demonstrates the low transfusion dependence in pts with PNH treated with eculizumab or ravulizumab, or pts switched to ravulizumab.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Transfusion
Abstract: EP1337
Type: E-Poster Presentation
Session title: Transfusion medicine
Background
Results from the International PNH Registry have shown that eculizumab reduced the number of transfusions by 50% in patients (pts) with PNH with transfusion history. Ravulizumab, the new standard of care for PNH, was non-inferior to eculizumab in 2 large clinical trials; 77% and 87% of pts treated with ravulizumab achieved transfusion avoidance at 1 year. Collecting real world data on the proportion of pts with PNH requiring transfusions whilst on ravulizumab, or after switching from eculizumab to ravulizumab (E-R switch) is important and valuable. Electronic medical records (EMR) and claims databases provide valuable real-world insight.
Aims
Determine proportion of pts with PNH receiving transfusions before and after initiating treatment with eculizumab, ravulizumab or E-R switch by using large-scale US EMR and claims databases.
Methods
TriNetX is a global health research network that integrates data from a variety of sources to create longitudinal real-world data, which can be sourced from EMR or claims. KOMODO Health is a comprehensive claims database in the US. The study population was identified using records with ICD-10 codes for PNH. Pts were grouped into three cohorts: received eculizumab but never ravulizumab (eculizumab-only), received ravulizumab but never eculizumab (ravulizumab-only), and an E-R switch. The TriNetX platform was used to access data up to 10/Feb/21 from two TriNetX networks: the US EMR and US Claims networks. Analyses for these databases were conducted at 6-month intervals: 6-months (mos) to 1 day pre-treatment, 0-6-mos post-treatment and continued at 6-month intervals thereafter until 18-24 mos in ravulizumab and E-R switch cohorts, and 42–48 mos in the eculizumab cohort. From the KOMODO Health database, claims data were sourced from 01/Jan/15 to 31/Dec/19. Analyses were conducted at 3-month intervals: 3 mos to 0-days pre-treatment, day of treatment (index date) to 3 mos post-treatment and at 3-month intervals until 6 mos for the ravulizumab and E-R switch cohorts, and 24 mos for the eculizumab cohort. Proportion of pts receiving transfusions at each interval was assessed.
Results
A total of 295, 804 and 231 pts with PNH that met the analysis criteria were identified from TriNetX EMR, TriNetX Claims and KOMODO, respectively (Figure 1). Proportion of pts receiving transfusions prior to index date in the eculizumab-only cohort was higher in all 3 databases. In the eculizumab-only cohort, the proportion of pts receiving transfusions decreased from 23–37% pre-treatment to 8–15% at the end of the study period. In the ravulizumab-only cohort, proportion of pts receiving transfusions decreased from 10–15% pre-treatment to 0% in the TriNetX Claims and KOMODO cohorts, and 8% in the TriNetX EMR cohort at the end of the study period. In the E-R switch cohort, proportion of pts receiving transfusions decreased from 9–17% pre-switch, to 0% in TriNetX Claims and TriNetX EMR cohorts and 8% in the KOMODO cohort at the end of study period after switching to ravulizumab. A limitation of this study was the small ravulizumab sample size.

Conclusion
Results of this real-world study demonstrate that the proportion of pts with PNH requiring blood transfusions was substantially reduced with eculizumab or ravulizumab monotherapy or after the E-R switch, with similar trends noted between treatment groups. This real-world data demonstrates the low transfusion dependence in pts with PNH treated with eculizumab or ravulizumab, or pts switched to ravulizumab.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Transfusion


