
Contributions
Abstract: EP1335
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Coagulopathy associated with COVID-19 is one of the main complications, especially in individuals with risk factors. Simultaneously, the use of low molecular weight heparin is the recommended strategy in hospitalized individuals, but the usefulness of other strategies such as DOACs in outpatients is still unknown.
Aims
Describe the benefit of the use of rivaroxaban at discharge on the risk of thrombosis in patients discharged from COVID-19.Identify the behavior of Dimero-D at discharge and its modification with anticoagulant therapy.
Methods
Randomized 1: 1 study (Rivaroxaban 10mg for 14 days versus observation) at discharge in individuals with COVID-19 who have not required mechanical ventilation. D-dimer was evaluated at 14 and 30 days after discharge in conjunction with a CT angiography at four weeks after hospital discharge. The protocol was developed in the Hospital Regional de Alta Especialidad de Ixtapaluca, authorized by the ethics committee with registration number NR-19-2020. The study is identified in ClinicalTrials.gov with the following registry NCT04508439
Results
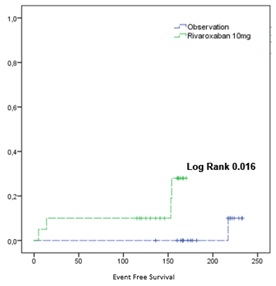
Forty individuals were evaluated (20 in each arm) with follow-up greater than 100 days. Gender distribution was equitable in each arm, with a mean age of 50 years (21 to 74 years), 40% (n = 16) were older than 55 years, 27.5% (n = 11) had hypertension and 20% (n = 8) had diabetes. The mean length of hospital stay was 10.5 days (6 to 23 days) treated with oxygen and low molecular weight heparin. At discharge, treatment was started with rivaroxaban 10mg x 14d vs. observation; in the rivaroxaban group, four events were identified (3 hemorrhages / 1 thrombosis), this difference being significant (Log Rank 0.016). Only one thrombosis event (pulmonary embolism) was identified in the rivaroxaban group at eight treatment days. Compared to the diagnosis, DD levels were lower at both 14 and 30 days (p = 0.000, 95% CI, 440.8-1164.9) without finding a difference in those treated with rivaroxaban or observation (p = 0.721, 95% CI, -68.48 to 241.18).
Conclusion
The use of DOACs at discharge does not benefit from an increased risk of hemorrhagic events; the anticoagulant strategy should be individualized, focused only on those patients at very high risk of thrombosis.
Keyword(s): Anticoagulation, COVID-19
Abstract: EP1335
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Coagulopathy associated with COVID-19 is one of the main complications, especially in individuals with risk factors. Simultaneously, the use of low molecular weight heparin is the recommended strategy in hospitalized individuals, but the usefulness of other strategies such as DOACs in outpatients is still unknown.
Aims
Describe the benefit of the use of rivaroxaban at discharge on the risk of thrombosis in patients discharged from COVID-19.Identify the behavior of Dimero-D at discharge and its modification with anticoagulant therapy.
Methods
Randomized 1: 1 study (Rivaroxaban 10mg for 14 days versus observation) at discharge in individuals with COVID-19 who have not required mechanical ventilation. D-dimer was evaluated at 14 and 30 days after discharge in conjunction with a CT angiography at four weeks after hospital discharge. The protocol was developed in the Hospital Regional de Alta Especialidad de Ixtapaluca, authorized by the ethics committee with registration number NR-19-2020. The study is identified in ClinicalTrials.gov with the following registry NCT04508439
Results
Forty individuals were evaluated (20 in each arm) with follow-up greater than 100 days. Gender distribution was equitable in each arm, with a mean age of 50 years (21 to 74 years), 40% (n = 16) were older than 55 years, 27.5% (n = 11) had hypertension and 20% (n = 8) had diabetes. The mean length of hospital stay was 10.5 days (6 to 23 days) treated with oxygen and low molecular weight heparin. At discharge, treatment was started with rivaroxaban 10mg x 14d vs. observation; in the rivaroxaban group, four events were identified (3 hemorrhages / 1 thrombosis), this difference being significant (Log Rank 0.016). Only one thrombosis event (pulmonary embolism) was identified in the rivaroxaban group at eight treatment days. Compared to the diagnosis, DD levels were lower at both 14 and 30 days (p = 0.000, 95% CI, 440.8-1164.9) without finding a difference in those treated with rivaroxaban or observation (p = 0.721, 95% CI, -68.48 to 241.18).

Conclusion
The use of DOACs at discharge does not benefit from an increased risk of hemorrhagic events; the anticoagulant strategy should be individualized, focused only on those patients at very high risk of thrombosis.
Keyword(s): Anticoagulation, COVID-19


