
Contributions
Abstract: EP1322
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Several observational studies have reported the rate of venous and arterial thrombotic events in patients infected with COVID-19, with conflicting results.
Aims
The aim of this study was to estimate the rate of thrombotic and bleeding events in hospitalized patients diagnosed with Coronavirus disease 2019 (COVID-19).
Methods
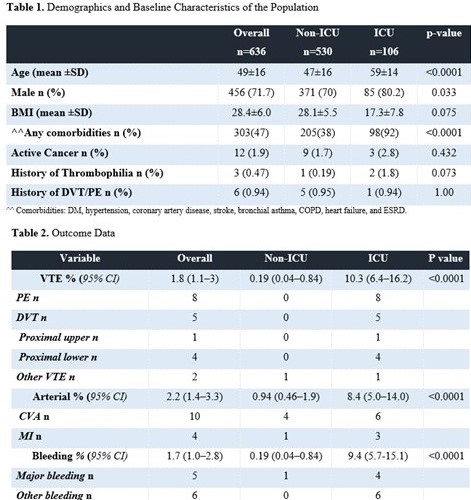
This was a multicenter study of 636 patients admitted between 20 March 2020 and 31 May 2020 with confirmed COVID-19 in four hospitals. Patients were excluded if they were transferred in or out from one of these four hospital to another hospitals. Patients also excluded is they were admitted for less than 24 hours. Venous thromboembolism (VTE) included all symptomatic or incidentally diagnosed cases of pulmonary embolism (PE), deep vein thrombosis (DVT) and thrombosis in unusual sites (cerebral, mesenteric, portal, splenic, hepatic, and renal veins). All VTEs were confirmed radiographically by appropriate imaging. Screening for VTE in asymptomatic patients was not performed. Arterial events included cerebrovascular accidents (CVAs), mesenteric ischemia, limb ischemia and myocardial infarction (MI) were confirmed by the appropriate clinical, laboratory and imaging modality. Bleeding events were classified as major and nonmajor based on the definition of International Society of Thrombosis and Haemostasis (ISTH). The rates of VTE, bleeding, arterial are summarized as proportions, with the corresponding 95% confidence intervals (CIs). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Informed consent was waived.
Results
The majority of patients (90%, n=573) and more than 99% of those in the ICU group received pharmacological prophylaxis according to local hospital practice. The most frequently prescribed regimen was enoxaparin (40 mg once daily) (59.2%). Over a median length of stay in the non-ICU group of 7 days and of 19 days in the ICU group, twelve patients were diagnosed with Venous thromboembolism (VTE) (1.8%) (95% CI, 1.1–3). The rate in the non-ICU group was 0.19% (95% CI, 0.04–0.84), and that in the ICU group was 10.3% (95% CI, 6.4–16.2).Two patients were not on prophylaxis prior to the event, 3 were on enoxaparin (40 mg once daily), 3 were on UFH (5000 IU twice daily), 2 were on UFH (5000 IU TID), one was on enoxaparin (40 mg twice daily), and one was on fondaparinux (2.5 mg once daily).The overall rate of arterial event is 2.2% (95% CI, 1.4–3.3). The rates in the non-ICU and ICU groups were 0.94% (95% CI, 0.46–0.1.9) and 8.4% (95% CI, 5.0–14.0). The overall rate of bleeding is 1.7% (95% CI, 1.0–2.8). The bleeding rate in the non-ICU group was 0.19% (95% CI, 0.04–0.84), and that in the ICU group was 9.4% (95% CI, 5.7–15.1). At the time of bleeding, six patients were on enoxparin 40 mg once, 2 were on UFH(Unfractionated heparin)5000 IU TID, 1 was on enoxparin 40 BID, 1 was on asprin and one was on full dose UFH.
Conclusion
In this study, we found that the VTE rates in hospitalized patients with COVID-19 might not be higher than expected. In contrast to the risk of VTE, we found a high rate of arterial and bleeding complications in patients admitted to the ICU. Given the high rate of bleeding, the current study suggests that the intensification of anticoagulation therapy in COVID-19 patients beyond the standard of care be pursued with caution and would best be evaluated in a randomized controlled study.
- Moores, Lisa K., et al. Chest 158.3 (2020): 1143-1163.
- Spyropoulos, Alex C., et al. Journal of Thrombosis and Haemostasis 18.8 (2020): 1859-1865.
Keyword(s): Bleeding, COVID-19, Stroke, Thrombosis
Abstract: EP1322
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Several observational studies have reported the rate of venous and arterial thrombotic events in patients infected with COVID-19, with conflicting results.
Aims
The aim of this study was to estimate the rate of thrombotic and bleeding events in hospitalized patients diagnosed with Coronavirus disease 2019 (COVID-19).
Methods
This was a multicenter study of 636 patients admitted between 20 March 2020 and 31 May 2020 with confirmed COVID-19 in four hospitals. Patients were excluded if they were transferred in or out from one of these four hospital to another hospitals. Patients also excluded is they were admitted for less than 24 hours. Venous thromboembolism (VTE) included all symptomatic or incidentally diagnosed cases of pulmonary embolism (PE), deep vein thrombosis (DVT) and thrombosis in unusual sites (cerebral, mesenteric, portal, splenic, hepatic, and renal veins). All VTEs were confirmed radiographically by appropriate imaging. Screening for VTE in asymptomatic patients was not performed. Arterial events included cerebrovascular accidents (CVAs), mesenteric ischemia, limb ischemia and myocardial infarction (MI) were confirmed by the appropriate clinical, laboratory and imaging modality. Bleeding events were classified as major and nonmajor based on the definition of International Society of Thrombosis and Haemostasis (ISTH). The rates of VTE, bleeding, arterial are summarized as proportions, with the corresponding 95% confidence intervals (CIs). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Informed consent was waived.
Results
The majority of patients (90%, n=573) and more than 99% of those in the ICU group received pharmacological prophylaxis according to local hospital practice. The most frequently prescribed regimen was enoxaparin (40 mg once daily) (59.2%). Over a median length of stay in the non-ICU group of 7 days and of 19 days in the ICU group, twelve patients were diagnosed with Venous thromboembolism (VTE) (1.8%) (95% CI, 1.1–3). The rate in the non-ICU group was 0.19% (95% CI, 0.04–0.84), and that in the ICU group was 10.3% (95% CI, 6.4–16.2).Two patients were not on prophylaxis prior to the event, 3 were on enoxaparin (40 mg once daily), 3 were on UFH (5000 IU twice daily), 2 were on UFH (5000 IU TID), one was on enoxaparin (40 mg twice daily), and one was on fondaparinux (2.5 mg once daily).The overall rate of arterial event is 2.2% (95% CI, 1.4–3.3). The rates in the non-ICU and ICU groups were 0.94% (95% CI, 0.46–0.1.9) and 8.4% (95% CI, 5.0–14.0). The overall rate of bleeding is 1.7% (95% CI, 1.0–2.8). The bleeding rate in the non-ICU group was 0.19% (95% CI, 0.04–0.84), and that in the ICU group was 9.4% (95% CI, 5.7–15.1). At the time of bleeding, six patients were on enoxparin 40 mg once, 2 were on UFH(Unfractionated heparin)5000 IU TID, 1 was on enoxparin 40 BID, 1 was on asprin and one was on full dose UFH.

Conclusion
In this study, we found that the VTE rates in hospitalized patients with COVID-19 might not be higher than expected. In contrast to the risk of VTE, we found a high rate of arterial and bleeding complications in patients admitted to the ICU. Given the high rate of bleeding, the current study suggests that the intensification of anticoagulation therapy in COVID-19 patients beyond the standard of care be pursued with caution and would best be evaluated in a randomized controlled study.
- Moores, Lisa K., et al. Chest 158.3 (2020): 1143-1163.
- Spyropoulos, Alex C., et al. Journal of Thrombosis and Haemostasis 18.8 (2020): 1859-1865.
Keyword(s): Bleeding, COVID-19, Stroke, Thrombosis


