Contributions
Abstract: EP1319
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Direct oral anticoagulants (DOACs) are widely used for the treatment and secondary prophylaxis of venous thromboembolism (VTE). Congenital thrombophilia is a condition that predisposes to a higher incidence of VTE, more frequent VTE recurrences, some also in atypical sites, and often require long-term anticoagulation for secondary prevention. It is less clear whether DOACs have the same effectiveness in patients with major thrombophilia than in non-thrombophilic patients for the prevention of VTE recurrences.
Aims
The aim of our study was to evaluate the efficacy, in terms of VTE prevention, and safety, in terms of absence of bleeding complications, in patients with major thrombophilia compared to non- thrombophilic patients candidate to long-term anticoagulation for recurrent VTE.
Methods
We evaluated consecutive patients who required long-term anticoagulation for recurrent VTE, treated with DOACs, and compared the outcomes between patients affected by major thrombophilia and non-thrombophilic patients. All patients presented at least 2 thrombotic events. Major thrombophilia was defined as the presence of physiologic inhibitors deficiency (protein C [PC], protein S [PS], and antithrombin (AT); homozygous Factor V Leiden, homozygous Factor II G20210A, combined heterozygosity of these defects. After DOACs start, patients were checked every 6 months for blood count, renal and liver function, coagulation, and for the onset of thrombotic and hemorrhagic adverse events.
Results
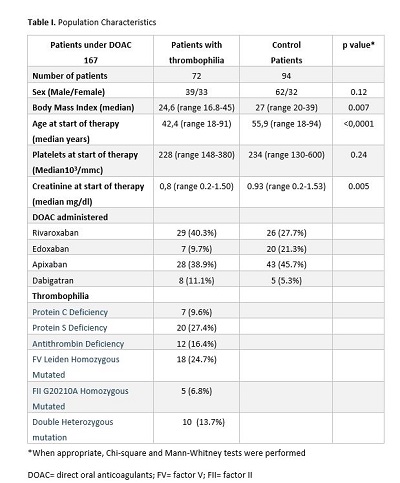
The examined patients were 167: 72 (43.4%) with major thrombophilia and 94 (66.6%) non-thrombophilic. Patients with major thrombophilia were younger than non-thrombophilic: median age 42 years (range 18-91 years) vs 56 years (range 18-94 years) (p <0.0001). All patients’ characteristics are specified in table 1. The median time of DOACS therapy was 32 months (range 6-90) in the group with major thrombophilia; 20 months (range 6-80) in the other group. Two (2.8%) thrombotic events were observed in the subset affected by major congenital thrombophilia and 4 (4.3%) in the non-thrombophilic group (p 0.4). Five (6.8%) minor bleedings were reported in the group of major thrombophilia; 13 (14%) hemorrhagic adverse events were observed in the other group: 10 minor and 3 clinical relevant non-major bleedings (p 0.14).
Conclusion
Although major thrombophilia predisposes to a higher incidence of VTE, our data suggest that in this setting of patients, DOACs are effective and safe, with comparable results to non-thrombophilic patients.
Keyword(s): Anticoagulation, Thrombophilia, Venous thromboembolism
Abstract: EP1319
Type: E-Poster Presentation
Session title: Thrombosis and vascular biology - Biology & Translational Research
Background
Direct oral anticoagulants (DOACs) are widely used for the treatment and secondary prophylaxis of venous thromboembolism (VTE). Congenital thrombophilia is a condition that predisposes to a higher incidence of VTE, more frequent VTE recurrences, some also in atypical sites, and often require long-term anticoagulation for secondary prevention. It is less clear whether DOACs have the same effectiveness in patients with major thrombophilia than in non-thrombophilic patients for the prevention of VTE recurrences.
Aims
The aim of our study was to evaluate the efficacy, in terms of VTE prevention, and safety, in terms of absence of bleeding complications, in patients with major thrombophilia compared to non- thrombophilic patients candidate to long-term anticoagulation for recurrent VTE.
Methods
We evaluated consecutive patients who required long-term anticoagulation for recurrent VTE, treated with DOACs, and compared the outcomes between patients affected by major thrombophilia and non-thrombophilic patients. All patients presented at least 2 thrombotic events. Major thrombophilia was defined as the presence of physiologic inhibitors deficiency (protein C [PC], protein S [PS], and antithrombin (AT); homozygous Factor V Leiden, homozygous Factor II G20210A, combined heterozygosity of these defects. After DOACs start, patients were checked every 6 months for blood count, renal and liver function, coagulation, and for the onset of thrombotic and hemorrhagic adverse events.
Results
The examined patients were 167: 72 (43.4%) with major thrombophilia and 94 (66.6%) non-thrombophilic. Patients with major thrombophilia were younger than non-thrombophilic: median age 42 years (range 18-91 years) vs 56 years (range 18-94 years) (p <0.0001). All patients’ characteristics are specified in table 1. The median time of DOACS therapy was 32 months (range 6-90) in the group with major thrombophilia; 20 months (range 6-80) in the other group. Two (2.8%) thrombotic events were observed in the subset affected by major congenital thrombophilia and 4 (4.3%) in the non-thrombophilic group (p 0.4). Five (6.8%) minor bleedings were reported in the group of major thrombophilia; 13 (14%) hemorrhagic adverse events were observed in the other group: 10 minor and 3 clinical relevant non-major bleedings (p 0.14).
Conclusion
Although major thrombophilia predisposes to a higher incidence of VTE, our data suggest that in this setting of patients, DOACs are effective and safe, with comparable results to non-thrombophilic patients.
Keyword(s): Anticoagulation, Thrombophilia, Venous thromboembolism