
Contributions
Abstract: EP1313
Type: E-Poster Presentation
Session title: Thalassemias
Background
In thalassemia major pancreatic iron was shown to be correlated to splenectomy, hepatitis C virus (HCV) infection, altered glucose metabolism, hepatic and cardiac iron, biventricular function, and myocardial fibrosis.
Aims
In the present multicenter study we explored the clinical correlates of pancreatic T2* in a cohort of patients with thalassemia intermedia (TI) under regular transfusion therapy.
Methods
We considered 150 patients (85 F, mean age 42.3±13.7 years) consecutively enrolled in the E-MIOT (Extension-Myocardial Iron Overload in Thalassemia) project. Magnetic Resonance Imaging (MRI) was performed to quantify hepatic, cardiac, and pancreatic iron overload by the T2* technique, to evaluate biventricular function parameters by cine images and to detect replacement myocardial fibrosis by late gadolinium enhancement (LGE) technique. To assess the disturbances of glucose metabolism, patients non already diagnosed with diabetes performed an oral glucose tolerance test (OGTT).
Results
Global pancreas T2* values were not correlated to age or gender. One-hundred and three patients (68.7%) showed a pathologic pancreas T2* value (<26 ms).
Splenectomised patients (76%) showed significantly lower global pancreas T2* than patients with the spleen (18.4±11.2 ms vs 23.8±13.4; P=0.017).
Patients with an active or eradicated HCV infection (34.9%) had significantly lower global pancreas T2* values than negative patients (17.1±10.6 ms vs 22.6±12.6; P=0.021).
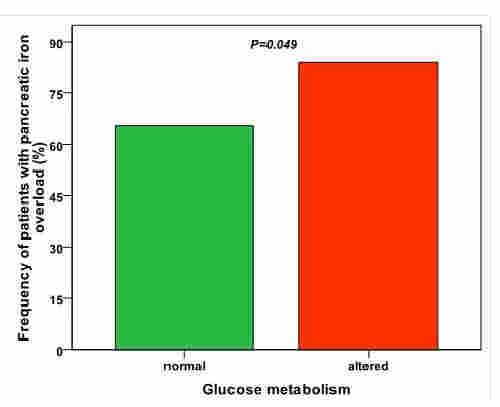
Patients with an altered OGTT (23%) showed a significant higher frequency of pancreatic iron overload than patients with a normal OGTT (83.9% vs 65.4%; P=0.049) (Figure 1). In non-diabetic patients global pancreatic T2* values were inversely correlated with glucose levels fasting (R=-0.366; P<0.0001) and 1-hr after OGTT (R=-0.308; P=0.016) and positively associated with the HOMA of β-cell function index (R=0.391; P=0.001).
Pancreas T2* values showed a negative correlation with MRI liver iron concentration values (R=-0.255; P=0.002) and a positive correlation with cardiac T2* values (R=0.184; P=0.024) and all the 6 patients with global heart T2*<20 ms had also pancreatic iron load.
Global pancreas T2* values were not correlated to biventricular volumes and ejection fraction and were comparable between patients without and with replacement myocardial fibrosis.
Conclusion
In regularly transfused TI patients higher pancreatic siderosis was found in splenectomised patients, in patients with an history of HCV infection, and in patients with altered glucose metabolism. Moreover, a normal pancreas T2* showed a negative predictive value of 100% for cardiac iron. These data support for starting or intensifying iron chelation therapy in TI patients with pancreatic iron overload. By this way it may be prospectively possible to prevent both alterations of glucose metabolism and cardiac iron accumulation rather than wait for the manifestation of overt diabetes and cardiac complications.
Keyword(s): Iron overload, Pancreas, Thalassemia
Abstract: EP1313
Type: E-Poster Presentation
Session title: Thalassemias
Background
In thalassemia major pancreatic iron was shown to be correlated to splenectomy, hepatitis C virus (HCV) infection, altered glucose metabolism, hepatic and cardiac iron, biventricular function, and myocardial fibrosis.
Aims
In the present multicenter study we explored the clinical correlates of pancreatic T2* in a cohort of patients with thalassemia intermedia (TI) under regular transfusion therapy.
Methods
We considered 150 patients (85 F, mean age 42.3±13.7 years) consecutively enrolled in the E-MIOT (Extension-Myocardial Iron Overload in Thalassemia) project. Magnetic Resonance Imaging (MRI) was performed to quantify hepatic, cardiac, and pancreatic iron overload by the T2* technique, to evaluate biventricular function parameters by cine images and to detect replacement myocardial fibrosis by late gadolinium enhancement (LGE) technique. To assess the disturbances of glucose metabolism, patients non already diagnosed with diabetes performed an oral glucose tolerance test (OGTT).
Results
Global pancreas T2* values were not correlated to age or gender. One-hundred and three patients (68.7%) showed a pathologic pancreas T2* value (<26 ms).
Splenectomised patients (76%) showed significantly lower global pancreas T2* than patients with the spleen (18.4±11.2 ms vs 23.8±13.4; P=0.017).
Patients with an active or eradicated HCV infection (34.9%) had significantly lower global pancreas T2* values than negative patients (17.1±10.6 ms vs 22.6±12.6; P=0.021).
Patients with an altered OGTT (23%) showed a significant higher frequency of pancreatic iron overload than patients with a normal OGTT (83.9% vs 65.4%; P=0.049) (Figure 1). In non-diabetic patients global pancreatic T2* values were inversely correlated with glucose levels fasting (R=-0.366; P<0.0001) and 1-hr after OGTT (R=-0.308; P=0.016) and positively associated with the HOMA of β-cell function index (R=0.391; P=0.001).
Pancreas T2* values showed a negative correlation with MRI liver iron concentration values (R=-0.255; P=0.002) and a positive correlation with cardiac T2* values (R=0.184; P=0.024) and all the 6 patients with global heart T2*<20 ms had also pancreatic iron load.
Global pancreas T2* values were not correlated to biventricular volumes and ejection fraction and were comparable between patients without and with replacement myocardial fibrosis.

Conclusion
In regularly transfused TI patients higher pancreatic siderosis was found in splenectomised patients, in patients with an history of HCV infection, and in patients with altered glucose metabolism. Moreover, a normal pancreas T2* showed a negative predictive value of 100% for cardiac iron. These data support for starting or intensifying iron chelation therapy in TI patients with pancreatic iron overload. By this way it may be prospectively possible to prevent both alterations of glucose metabolism and cardiac iron accumulation rather than wait for the manifestation of overt diabetes and cardiac complications.
Keyword(s): Iron overload, Pancreas, Thalassemia


