
Contributions
Abstract: EP1312
Type: E-Poster Presentation
Session title: Thalassemias
Background
Adherence with life-long iron chelation therapy is critical for the survival of transfusion-dependent patients. Deferiprone is an orally absorbed bidentate hydroxypyridinone iron chelator with high efficacy in binding excess intracellular iron to reduce iron toxicity and is approved for the treatment of iron overload. The current formulation of deferiprone is immediate release (IR) and requires three-times-daily (TID) administration, which may result in decreased adherence in some patients.
Aims
A twice-daily (BID) deferiprone formulation was developed as a more convenient dosing regimen to improve patient adherence. In the current study, we evaluated the safety, tolerability, and acceptability of deferiprone BID tablets in a cohort of patients with transfusion-dependent thalassemia (TDT).
Methods
TWICE (NCT03802916) was a multicenter, open-label, switch-over study of 30 adult patients with TDT who were taking deferiprone IR TID, and the first study where patients with iron overload were administered deferiprone BID. The currently approved dosage of deferiprone IR TID is 75-99 mg/kg/d. To assess the impact of the BID formulation within the approved dosage range on adverse events (AEs), an equal number of patients were enrolled into either a “low-end standard dose” (LSD; ~75 mg/kg/d) or “high-end standard dose” (HSD; ~100 mg/kg/d) group based on their deferiprone IR TID dosage. At the end-of-study visit (day 28 of treatment), patients completed a preference questionnaire on the acceptability of the BID versus the IR TID formulation.
Results
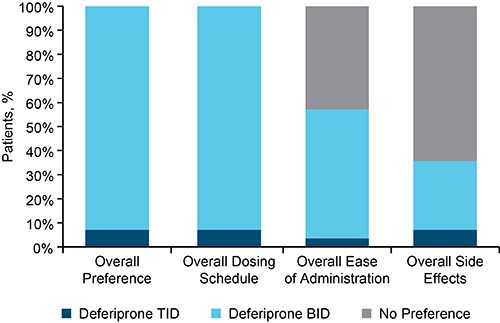
The study enrolled 30 patients with TDT; 1 patient in the HSD group withdrew before receiving deferiprone BID. The study population consisted of 29 participants (44.8% female) with a mean age of 41.1 (SD: 8.4) years. Nineteen patients (10 in the LSD and 9 in the HSD group), experienced a total of 49 AEs; 41% were classified as mild, 57% moderate, and 2% severe. The most common AEs, irrespective of a causal relationship with therapy, were headache (21%), arthralgia (14%), and diarrhea (10%). 13 AEs (27%) were considered “at least possibly related” to therapy; those which were reported in 2 or more patients included diarrhea (n=3, HSD) and arthralgia (n=1, LSD; n=1, HSD). One patient in the LSD group experienced a severe AE of arthralgia (elbow pain) that resolved without interruption of therapy. All episodes of diarrhea were transient and resolved without interruption of therapy. One patient in the HSD group withdrew after experiencing a mild AE of renal colic, which was considered “possibly related” to therapy. Serious AEs previously ascribed to deferiprone IR TID (ie, agranulocytosis, neutropenia, or clinically concerning increases in liver enzymes) were not observed with deferiprone BID. The mean adherence to the deferiprone BID dosing regimen was 99.3% in the LSD and 98.5% in the HSD group. At the end-of-study visit, most patients (92.9%) indicated their strong preference for the deferiprone BID formulation over the IR TID formulation (Figure).
Conclusion
The new formulation of deferiprone BID tablets was safe and well-tolerated among patients with TDT. Most AEs were mild or moderate, and none of the serious AEs previously observed with deferiprone IR TID were reported. Patients reported high adherence to the deferiprone BID formulation and most (92.9%) preferred the deferiprone BID formulation over the IR TID, suggesting the BID formulation has the potential to improve patient adherence.
Keyword(s): Anemia, Deferiprone, Iron chelation, Iron overload
Abstract: EP1312
Type: E-Poster Presentation
Session title: Thalassemias
Background
Adherence with life-long iron chelation therapy is critical for the survival of transfusion-dependent patients. Deferiprone is an orally absorbed bidentate hydroxypyridinone iron chelator with high efficacy in binding excess intracellular iron to reduce iron toxicity and is approved for the treatment of iron overload. The current formulation of deferiprone is immediate release (IR) and requires three-times-daily (TID) administration, which may result in decreased adherence in some patients.
Aims
A twice-daily (BID) deferiprone formulation was developed as a more convenient dosing regimen to improve patient adherence. In the current study, we evaluated the safety, tolerability, and acceptability of deferiprone BID tablets in a cohort of patients with transfusion-dependent thalassemia (TDT).
Methods
TWICE (NCT03802916) was a multicenter, open-label, switch-over study of 30 adult patients with TDT who were taking deferiprone IR TID, and the first study where patients with iron overload were administered deferiprone BID. The currently approved dosage of deferiprone IR TID is 75-99 mg/kg/d. To assess the impact of the BID formulation within the approved dosage range on adverse events (AEs), an equal number of patients were enrolled into either a “low-end standard dose” (LSD; ~75 mg/kg/d) or “high-end standard dose” (HSD; ~100 mg/kg/d) group based on their deferiprone IR TID dosage. At the end-of-study visit (day 28 of treatment), patients completed a preference questionnaire on the acceptability of the BID versus the IR TID formulation.
Results
The study enrolled 30 patients with TDT; 1 patient in the HSD group withdrew before receiving deferiprone BID. The study population consisted of 29 participants (44.8% female) with a mean age of 41.1 (SD: 8.4) years. Nineteen patients (10 in the LSD and 9 in the HSD group), experienced a total of 49 AEs; 41% were classified as mild, 57% moderate, and 2% severe. The most common AEs, irrespective of a causal relationship with therapy, were headache (21%), arthralgia (14%), and diarrhea (10%). 13 AEs (27%) were considered “at least possibly related” to therapy; those which were reported in 2 or more patients included diarrhea (n=3, HSD) and arthralgia (n=1, LSD; n=1, HSD). One patient in the LSD group experienced a severe AE of arthralgia (elbow pain) that resolved without interruption of therapy. All episodes of diarrhea were transient and resolved without interruption of therapy. One patient in the HSD group withdrew after experiencing a mild AE of renal colic, which was considered “possibly related” to therapy. Serious AEs previously ascribed to deferiprone IR TID (ie, agranulocytosis, neutropenia, or clinically concerning increases in liver enzymes) were not observed with deferiprone BID. The mean adherence to the deferiprone BID dosing regimen was 99.3% in the LSD and 98.5% in the HSD group. At the end-of-study visit, most patients (92.9%) indicated their strong preference for the deferiprone BID formulation over the IR TID formulation (Figure).

Conclusion
The new formulation of deferiprone BID tablets was safe and well-tolerated among patients with TDT. Most AEs were mild or moderate, and none of the serious AEs previously observed with deferiprone IR TID were reported. Patients reported high adherence to the deferiprone BID formulation and most (92.9%) preferred the deferiprone BID formulation over the IR TID, suggesting the BID formulation has the potential to improve patient adherence.
Keyword(s): Anemia, Deferiprone, Iron chelation, Iron overload


