
Contributions
Abstract: EP1306
Type: E-Poster Presentation
Session title: Thalassemias
Background
β-thalassemia is characterized by anemia and ineffective erythropoiesis. Appropriate schemes of RBC transfusions (RBCT) are the backbone of effective anemia treatment, but at the cost of progressive iron overload and iron-related complications. In the BELIEVE trial (NCT02604433), a phase 3, double-blind, multicenter, placebo-controlled study, the safety and efficacy of luspatercept, a first-in-class erythroid maturation agent, was evaluated in adult patients (pts) with β-thalassemia receiving RBCT. Luspatercept significantly reduced TB vs placebo (primary endpoint ≥33% reduction in RBCT in Weeks (Wks) 13–24 from baseline [≥2 RBC units]). As per protocol, pts that did not fully achieve the primary endpoint were defined as non-responders. In this group, the potential clinical benefit of treatment continuation with luspatercept has not yet been explored.
Aims
To evaluate the treatment benefit of continuing luspatercept therapy in pts who did not achieve the primary endpoint.
Methods
The BELIEVE trial enrolled pts aged ≥18 years with β-thalassemia or hemoglobin (Hb) E/β‑thalassemia (including compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBCT (6–20 RBC units in the 24 wks prior to randomization, no RBCT-free period >35 days). Pts were randomized 2:1 to luspatercept 1.0 mg/kg (up to 1.25 mg/kg allowed) or placebo subcutaneously every 3 wks for 48 wks during the double-blind period. Pts in both treatment arms continued to receive best supportive care, including RBCT to maintain target Hb levels and iron chelation therapy adjusted to iron overload level. In this analysis, pts who did not achieve the primary endpoint during Wks 13–24 were followed until Wk 48. The data cutoff for this analysis was July 1, 2019.
Results
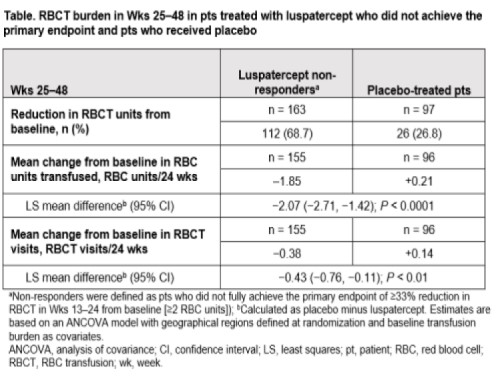
Of 224 pts who received luspatercept, 177 (79%) did not fully achieve the primary endpoint and were defined as non-responders. Of 163 luspatercept non-responders who continued treatment in Wks 25−48, 112 (68.7%) achieved a subsequent reduction in RBCT units from baseline vs 26/97 (26.8%) pts in the placebo arm (Table). For RBC units transfused, luspatercept non-responders had a mean change from baseline of −1.85 RBC units/24 wks vs +0.21 RBC units/24 wks in placebo-treated pts during Wks 25–48. For RBCT visits, luspatercept non-responders experienced a mean change of −0.38 RBCT visits/24 wks vs +0.14 RBCT visits/24 wks in placebo-treated pts during Wks 25–48.
In any 12-wk period during Wks 1–48, a reduction of ≥33% RBCT units from baseline was achieved by 119/163 (73.0%) luspatercept non-responders vs 33/97 (34.0%) pts in the placebo arm. A reduction of ≥50% RBCT units from baseline was achieved by 62/163 (38.0%) luspatercept non-responders vs 6/97 (6.2%) pts in the placebo arm.
In Wks 25–48, 97/163 (59.5%) luspatercept non-responders achieved a reduction in serum ferritin (SF) from baseline vs 36/97 (37.1%) pts in the placebo arm. For non-responders, the mean change in SF from baseline was −203.34 mg/L (−8.07%). Of 103 non-responders with baseline mean SF ≥1,000 μg/L, 20 (19.4%) achieved post-baseline mean SF <1,000 μg/L in Wks 25–48.
Conclusion
Most pts with β-thalassemia who did not meet the primary endpoint during the BELIEVE trial and were defined as non-responders to luspatercept had clinical benefit from continuing therapy. Pts received fewer RBC units, had fewer hospital visits due to RBCT administration, and had lower SF levels.
Keyword(s): Beta thalassemia, Clinical trial
Abstract: EP1306
Type: E-Poster Presentation
Session title: Thalassemias
Background
β-thalassemia is characterized by anemia and ineffective erythropoiesis. Appropriate schemes of RBC transfusions (RBCT) are the backbone of effective anemia treatment, but at the cost of progressive iron overload and iron-related complications. In the BELIEVE trial (NCT02604433), a phase 3, double-blind, multicenter, placebo-controlled study, the safety and efficacy of luspatercept, a first-in-class erythroid maturation agent, was evaluated in adult patients (pts) with β-thalassemia receiving RBCT. Luspatercept significantly reduced TB vs placebo (primary endpoint ≥33% reduction in RBCT in Weeks (Wks) 13–24 from baseline [≥2 RBC units]). As per protocol, pts that did not fully achieve the primary endpoint were defined as non-responders. In this group, the potential clinical benefit of treatment continuation with luspatercept has not yet been explored.
Aims
To evaluate the treatment benefit of continuing luspatercept therapy in pts who did not achieve the primary endpoint.
Methods
The BELIEVE trial enrolled pts aged ≥18 years with β-thalassemia or hemoglobin (Hb) E/β‑thalassemia (including compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBCT (6–20 RBC units in the 24 wks prior to randomization, no RBCT-free period >35 days). Pts were randomized 2:1 to luspatercept 1.0 mg/kg (up to 1.25 mg/kg allowed) or placebo subcutaneously every 3 wks for 48 wks during the double-blind period. Pts in both treatment arms continued to receive best supportive care, including RBCT to maintain target Hb levels and iron chelation therapy adjusted to iron overload level. In this analysis, pts who did not achieve the primary endpoint during Wks 13–24 were followed until Wk 48. The data cutoff for this analysis was July 1, 2019.
Results
Of 224 pts who received luspatercept, 177 (79%) did not fully achieve the primary endpoint and were defined as non-responders. Of 163 luspatercept non-responders who continued treatment in Wks 25−48, 112 (68.7%) achieved a subsequent reduction in RBCT units from baseline vs 26/97 (26.8%) pts in the placebo arm (Table). For RBC units transfused, luspatercept non-responders had a mean change from baseline of −1.85 RBC units/24 wks vs +0.21 RBC units/24 wks in placebo-treated pts during Wks 25–48. For RBCT visits, luspatercept non-responders experienced a mean change of −0.38 RBCT visits/24 wks vs +0.14 RBCT visits/24 wks in placebo-treated pts during Wks 25–48.
In any 12-wk period during Wks 1–48, a reduction of ≥33% RBCT units from baseline was achieved by 119/163 (73.0%) luspatercept non-responders vs 33/97 (34.0%) pts in the placebo arm. A reduction of ≥50% RBCT units from baseline was achieved by 62/163 (38.0%) luspatercept non-responders vs 6/97 (6.2%) pts in the placebo arm.
In Wks 25–48, 97/163 (59.5%) luspatercept non-responders achieved a reduction in serum ferritin (SF) from baseline vs 36/97 (37.1%) pts in the placebo arm. For non-responders, the mean change in SF from baseline was −203.34 mg/L (−8.07%). Of 103 non-responders with baseline mean SF ≥1,000 μg/L, 20 (19.4%) achieved post-baseline mean SF <1,000 μg/L in Wks 25–48.

Conclusion
Most pts with β-thalassemia who did not meet the primary endpoint during the BELIEVE trial and were defined as non-responders to luspatercept had clinical benefit from continuing therapy. Pts received fewer RBC units, had fewer hospital visits due to RBCT administration, and had lower SF levels.
Keyword(s): Beta thalassemia, Clinical trial


