
Contributions
Abstract: EP1304
Type: E-Poster Presentation
Session title: Thalassemias
Background
β-thalassemia is a genetic blood disorder marked by ineffective erythropoiesis and anemia. The first-in-class erythroid maturation agent, luspatercept, enhances late-stage erythroid hematopoiesis. Luspatercept was shown to be effective in reducing red blood cell (RBC) transfusion burden (TB) in adult patients (pts) with β-thalassemia requiring regular RBC transfusions in the phase 3, double-blind, randomized, placebo-controlled BELIEVE trial (NCT02604433). The effect of luspatercept treatment on RBC TB warrants further investigation.
Aims
To assess the mean cumulative number of RBC transfusion visits and units across baseline (BL) low, medium, and high TB levels among pts with β-thalassemia in the BELIEVE trial.
Methods
Eligible pts were aged ≥18 years with β-thalassemia or hemoglobin (Hb) E/β‑thalassemia (compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBC transfusions (defined as 6–20 RBC units in the 24 weeks (wks) prior to randomization with no transfusion-free period >35 days). Pts were randomized 2:1 to luspatercept 1.0 mg/kg (up to 1.25 mg/kg allowed) or placebo subcutaneously every 3 wks for 48 wks during the double-blind period. After study unblinding, pts randomized to placebo were eligible to cross over to receive luspatercept in an open-label phase.
Luspatercept responders (n = 47; 21%) were defined as pts who achieved the primary endpoint of RBC TB reduction of ≥33% during Wks 13–24 from BL (≥2 RBC units). BL low, medium, and high TB were defined as receipt of ≤10, >10–≤15, and >15 RBC units/24 wks, respectively. The mean cumulative number of RBC transfusion units and visits was assessed per pt in luspatercept responders and placebo-treated pts up to Wk 48, and in luspatercept responders and non-responders up to Wk 120, excluding pts who crossed over from placebo. Cumulative mean numbers of RBC transfusion units and visits were estimated using the Nelson-Aalen nonparametric estimator with robust variance estimate. The data cutoff was July 1, 2019.
Results
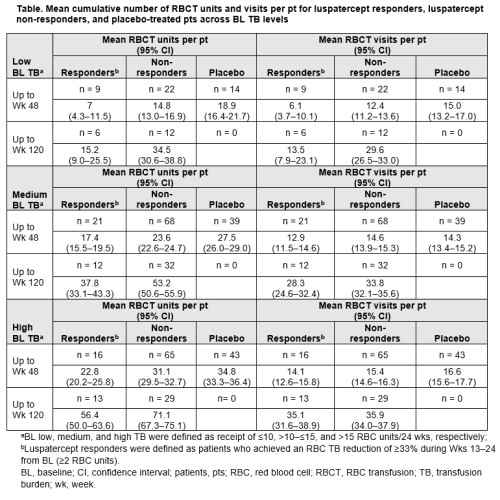
Luspatercept responders had fewer RBC units transfused vs placebo up to Wk 48 and vs luspatercept non-responders up to Wk 120, across BL TB levels (Table). Up to Wk 120, the mean cumulative number (95% confidence interval [CI]) of RBC units transfused per pt for luspatercept responders vs non-responders was 15.2 (9.0–25.5) vs 34.5 (30.6–38.8) for low BL TB, 37.8 (33.1–43.3) vs 53.2 (50.6–55.9) for medium BL TB, and 56.4 (50.0–63.6) vs 71.1 (67.3–75.1) for high BL TB.
Luspatercept responders also had fewer RBC transfusion visits vs placebo up to Wk 48 and vs luspatercept non-responders up to Wk 120, regardless of TB at BL (Table). Up to Wk 120, the mean cumulative number (95% CI) of RBC transfusion visits per pt for luspatercept responders vs non-responders was 13.5 (7.9–23.1) and 29.6 (26.5–33.0) for low BL TB, 28.3 (24.6–32.4) and 33.8 (32.1–35.6) for medium BL TB, and 35.1 (31.6–38.9) and 35.9 (34.0–37.9.) for high BL TB.
Conclusion
These findings show that luspatercept responders in the BELIEVE trial had reduced numbers of RBC units transfused and transfusion visits over 120 wks, regardless of BL TB. The observed reduction in TB in pts with β-thalassemia treated with luspatercept may have a positive impact on clinical and economic outcomes.
Keyword(s): Beta thalassemia, Clinical trial
Abstract: EP1304
Type: E-Poster Presentation
Session title: Thalassemias
Background
β-thalassemia is a genetic blood disorder marked by ineffective erythropoiesis and anemia. The first-in-class erythroid maturation agent, luspatercept, enhances late-stage erythroid hematopoiesis. Luspatercept was shown to be effective in reducing red blood cell (RBC) transfusion burden (TB) in adult patients (pts) with β-thalassemia requiring regular RBC transfusions in the phase 3, double-blind, randomized, placebo-controlled BELIEVE trial (NCT02604433). The effect of luspatercept treatment on RBC TB warrants further investigation.
Aims
To assess the mean cumulative number of RBC transfusion visits and units across baseline (BL) low, medium, and high TB levels among pts with β-thalassemia in the BELIEVE trial.
Methods
Eligible pts were aged ≥18 years with β-thalassemia or hemoglobin (Hb) E/β‑thalassemia (compound β-thalassemia mutation and/or multiplication of α-globin genes was allowed) and required regular RBC transfusions (defined as 6–20 RBC units in the 24 weeks (wks) prior to randomization with no transfusion-free period >35 days). Pts were randomized 2:1 to luspatercept 1.0 mg/kg (up to 1.25 mg/kg allowed) or placebo subcutaneously every 3 wks for 48 wks during the double-blind period. After study unblinding, pts randomized to placebo were eligible to cross over to receive luspatercept in an open-label phase.
Luspatercept responders (n = 47; 21%) were defined as pts who achieved the primary endpoint of RBC TB reduction of ≥33% during Wks 13–24 from BL (≥2 RBC units). BL low, medium, and high TB were defined as receipt of ≤10, >10–≤15, and >15 RBC units/24 wks, respectively. The mean cumulative number of RBC transfusion units and visits was assessed per pt in luspatercept responders and placebo-treated pts up to Wk 48, and in luspatercept responders and non-responders up to Wk 120, excluding pts who crossed over from placebo. Cumulative mean numbers of RBC transfusion units and visits were estimated using the Nelson-Aalen nonparametric estimator with robust variance estimate. The data cutoff was July 1, 2019.
Results
Luspatercept responders had fewer RBC units transfused vs placebo up to Wk 48 and vs luspatercept non-responders up to Wk 120, across BL TB levels (Table). Up to Wk 120, the mean cumulative number (95% confidence interval [CI]) of RBC units transfused per pt for luspatercept responders vs non-responders was 15.2 (9.0–25.5) vs 34.5 (30.6–38.8) for low BL TB, 37.8 (33.1–43.3) vs 53.2 (50.6–55.9) for medium BL TB, and 56.4 (50.0–63.6) vs 71.1 (67.3–75.1) for high BL TB.
Luspatercept responders also had fewer RBC transfusion visits vs placebo up to Wk 48 and vs luspatercept non-responders up to Wk 120, regardless of TB at BL (Table). Up to Wk 120, the mean cumulative number (95% CI) of RBC transfusion visits per pt for luspatercept responders vs non-responders was 13.5 (7.9–23.1) and 29.6 (26.5–33.0) for low BL TB, 28.3 (24.6–32.4) and 33.8 (32.1–35.6) for medium BL TB, and 35.1 (31.6–38.9) and 35.9 (34.0–37.9.) for high BL TB.

Conclusion
These findings show that luspatercept responders in the BELIEVE trial had reduced numbers of RBC units transfused and transfusion visits over 120 wks, regardless of BL TB. The observed reduction in TB in pts with β-thalassemia treated with luspatercept may have a positive impact on clinical and economic outcomes.
Keyword(s): Beta thalassemia, Clinical trial


