
Contributions
Abstract: EP1289
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Haploidentical stem cell transplants (Haplo-SCT) with post-transplant cyclophosphamide (PTCy) are increasing in application due to donor availability and simplicity. However, factors predictive of non-relapse mortality (NRM) in PTCy remain relatively poorly defined.
Aims
To evaluate factors predictive of NRM in patients undergoing T-replete PTCy Haplo-SCT at our centre.
Methods
Consecutive patients undertaking PTCy Haplo-SCT between January 2017 and October 2020 were identified from an institutional database and outcomes retrospectively determined from review of individual medical records. Conditioning regimens included myeloablative (MAB; Fludarabine / 12Gy TBI), reduced intensity (RIC; Melphalan/ Fludarabine/ 2Gy TBI) and non-myeloablative (NMA; Fludarabine / Cyclophosphamide / 2Gy TBI +/- rabbit ATG) regimens. All grafts were T-replete PBPC with patients commencing immunosuppression with MMF and tacrolimus post completion of PTCy.
Results
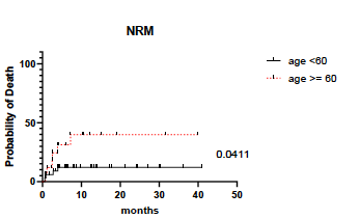
51 patients underwent PTCy Haplo-SCT with PTCy during the time period under review. Median age at transplant was 50yrs (range 19-74yrs), with 18 patients (35%) aged >60yrs. Indication for SCT included AML in 26 patients (51%), MDS/ MPN in 10 (20%), B-ALL in 7 (14%), LPD in 6 (12%) and SAA in 2 (4%). Conditioning regimens were MAB in 16 (31%), RIC in 25 (49%) and NMA in 10 (20%). Haplo donors utilized included parents in 7 (14%), siblings in 15 (29%) and children in 29 (57%). Overall incidence of grade II-IV and III-IV acute GVHD was 27% and 14% respectively, with 12% of patients surviving post D100 developing extensive stage chronic GVHD. At median follow-up for survivors of 12mths 1yr OS, PFS, and NRM was 76%, 67%, and 21% respectively. In univariate analyses, factors associated with NRM included recipient age (>60yrs) and occurrence of ICANS post PBPC infusion, which occurred in 8 (16%) of patients (n=4 > grade 3). Recipient age (>60yrs) remained predictive of NRM, with NRM of 12% in patients <60yrs vs 40% in those >60yrs respectively (p=0.041; see Figure). Conditioning regimen and HCT-CI score did not predict for NRM.
Conclusion
PTCy Haplo-SCT is associated with significant NRM in patients >60yrs. Further studies evaluating less toxic regimens is needed for this group of patients.
Keyword(s): Haploidentical stem cell transplantation, Post-transplant
Abstract: EP1289
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Haploidentical stem cell transplants (Haplo-SCT) with post-transplant cyclophosphamide (PTCy) are increasing in application due to donor availability and simplicity. However, factors predictive of non-relapse mortality (NRM) in PTCy remain relatively poorly defined.
Aims
To evaluate factors predictive of NRM in patients undergoing T-replete PTCy Haplo-SCT at our centre.
Methods
Consecutive patients undertaking PTCy Haplo-SCT between January 2017 and October 2020 were identified from an institutional database and outcomes retrospectively determined from review of individual medical records. Conditioning regimens included myeloablative (MAB; Fludarabine / 12Gy TBI), reduced intensity (RIC; Melphalan/ Fludarabine/ 2Gy TBI) and non-myeloablative (NMA; Fludarabine / Cyclophosphamide / 2Gy TBI +/- rabbit ATG) regimens. All grafts were T-replete PBPC with patients commencing immunosuppression with MMF and tacrolimus post completion of PTCy.
Results
51 patients underwent PTCy Haplo-SCT with PTCy during the time period under review. Median age at transplant was 50yrs (range 19-74yrs), with 18 patients (35%) aged >60yrs. Indication for SCT included AML in 26 patients (51%), MDS/ MPN in 10 (20%), B-ALL in 7 (14%), LPD in 6 (12%) and SAA in 2 (4%). Conditioning regimens were MAB in 16 (31%), RIC in 25 (49%) and NMA in 10 (20%). Haplo donors utilized included parents in 7 (14%), siblings in 15 (29%) and children in 29 (57%). Overall incidence of grade II-IV and III-IV acute GVHD was 27% and 14% respectively, with 12% of patients surviving post D100 developing extensive stage chronic GVHD. At median follow-up for survivors of 12mths 1yr OS, PFS, and NRM was 76%, 67%, and 21% respectively. In univariate analyses, factors associated with NRM included recipient age (>60yrs) and occurrence of ICANS post PBPC infusion, which occurred in 8 (16%) of patients (n=4 > grade 3). Recipient age (>60yrs) remained predictive of NRM, with NRM of 12% in patients <60yrs vs 40% in those >60yrs respectively (p=0.041; see Figure). Conditioning regimen and HCT-CI score did not predict for NRM.

Conclusion
PTCy Haplo-SCT is associated with significant NRM in patients >60yrs. Further studies evaluating less toxic regimens is needed for this group of patients.
Keyword(s): Haploidentical stem cell transplantation, Post-transplant


