
Contributions
Abstract: EP1277
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
The use of Bcl-2 inhibitor venetoclax (Ven) combined with hypomethylating agents or chemotherapy has shown promising results in treating Acute myeloid leukemia (AML) and other hematologic malignancies as frontline treatment and for relapse. However, studies regarding the effect of Ven therapy on subsequent HSCT are limited.
Aims
Aiming to evaluate the outcome of patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) after Ven therapy, we retrospectively collected data from patients treated at four transplant centers in Zhejiang Province, China.
Methods
Patients who had received ≥1 cycle of Ven combinations prior to undergoing HSCT were included. Conditioning regimens included myeloablative regimens and nonmyeloablative regimens, which were determined by the individual transplant physician based upon disease- and transplant-related considerations.
Results
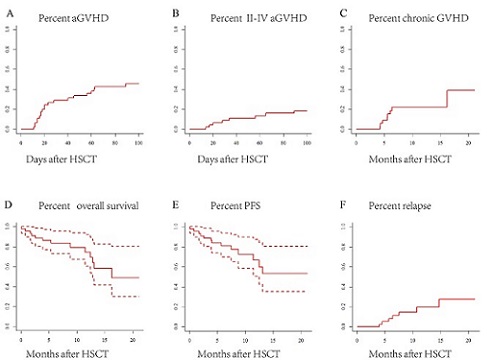
Forty-five patients who underwent HSCT after Ven therapy were enrolled between November 2018 and November 2020. Thirty-one patients were diagnosed with AML (28 were de novo AML, 3 were secondary for MDS), 6 with MDS,3 with ALL, 2 with CMML, and 2 others. The majority (75.6%) of patients received Ven for treatment of relapse (40.0%) or induction failure (35.6%), 5(11.1%) were treated as initial treatment, and 6(13.3%) patients who were already in CR received Ven for further consolidation or deep remission before HSCT. Thirty-three (73.3%) patients were in complete remission at the time of HSCT. The median time of neutropenia and platelet engraftment were 12 days and 13 days, respectively. Day +100 cumulative incidences of acute graft-vs-host disease (aGVHD) and grade II-IV aGVHD was 42.9% and 15.9%. Of 39 evaluable patients, 38.9% developed chronic GVHD developed. The 100-day cytomegalovirus (CMV) reactivation occurred in 74.4% of patients; Epstein-Barr virus (EBV) reactivation in 38.1% of patients. With a median follow-up of 9.5 months, one-year overall survival, progression-free survival and relapse incidence were 74.5%, 56.9% and 19.8%, respectively.
Conclusion
Our multicenter results showed that patients receiving HSCT after Ven therapy had favorable transplant outcomes with low aGVHD and relapse incidence, suggesting the feasibility of Ven proceeding to HSCT. Meanwhile, virus reactivation monitor should be paid attention.
Keyword(s): Hematopoietic cell transplantation
Abstract: EP1277
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
The use of Bcl-2 inhibitor venetoclax (Ven) combined with hypomethylating agents or chemotherapy has shown promising results in treating Acute myeloid leukemia (AML) and other hematologic malignancies as frontline treatment and for relapse. However, studies regarding the effect of Ven therapy on subsequent HSCT are limited.
Aims
Aiming to evaluate the outcome of patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) after Ven therapy, we retrospectively collected data from patients treated at four transplant centers in Zhejiang Province, China.
Methods
Patients who had received ≥1 cycle of Ven combinations prior to undergoing HSCT were included. Conditioning regimens included myeloablative regimens and nonmyeloablative regimens, which were determined by the individual transplant physician based upon disease- and transplant-related considerations.
Results
Forty-five patients who underwent HSCT after Ven therapy were enrolled between November 2018 and November 2020. Thirty-one patients were diagnosed with AML (28 were de novo AML, 3 were secondary for MDS), 6 with MDS,3 with ALL, 2 with CMML, and 2 others. The majority (75.6%) of patients received Ven for treatment of relapse (40.0%) or induction failure (35.6%), 5(11.1%) were treated as initial treatment, and 6(13.3%) patients who were already in CR received Ven for further consolidation or deep remission before HSCT. Thirty-three (73.3%) patients were in complete remission at the time of HSCT. The median time of neutropenia and platelet engraftment were 12 days and 13 days, respectively. Day +100 cumulative incidences of acute graft-vs-host disease (aGVHD) and grade II-IV aGVHD was 42.9% and 15.9%. Of 39 evaluable patients, 38.9% developed chronic GVHD developed. The 100-day cytomegalovirus (CMV) reactivation occurred in 74.4% of patients; Epstein-Barr virus (EBV) reactivation in 38.1% of patients. With a median follow-up of 9.5 months, one-year overall survival, progression-free survival and relapse incidence were 74.5%, 56.9% and 19.8%, respectively.

Conclusion
Our multicenter results showed that patients receiving HSCT after Ven therapy had favorable transplant outcomes with low aGVHD and relapse incidence, suggesting the feasibility of Ven proceeding to HSCT. Meanwhile, virus reactivation monitor should be paid attention.
Keyword(s): Hematopoietic cell transplantation


