
Contributions
Abstract: EP1276
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Haploidentical haematopoietic stem cell transplant (haplo-HSCT) is a potentially curative option for patients with myeloid malignancies lacking an HLA identical donor. Although the original platform was based on bone marrow (BM) as cellular source, many transplant centres now use G-CSF mobilised peripheral blood stem cells (PBSC). Chronic graft versus host disease (cGVHD) is a complication that can jeopardize the transplant outcome; for that reason, some centres still prefer BM or use t-depletion with ATG. Relapse rates are also of concern, and the optimal intensity of conditioning (MA vs RIC) in haplo-HSCT for patient with acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) remains unclear.
Aims
The aim of this paper is to compare results of two different haplo-HSCT approaches (BM vs PBSC and MA vs RIC) in patients affected by AML and MDS at King’s College Hospital (KCH) in London and King Faisal Hospital (KFH) in Riyadh.
Methods
Between August 2010 and March 2020, 50 patients with a median age of 46 (14-60yrs) and a high-risk myeloid malignancy were identified (AML n=34, MDS n=16). At KCH, haplo-HSCT (n=23) was performed with G-CSF mobilized PBSC with FluCyTBI protocol (Cy 14.5 mg/kg/ days -6, -5, fludarabine 30 mg/m2/ days -6 to -2, and 200 cGy Total Body Irradiation day -1). At KFH 27 patients had t-deplete BM with TBF regimen (thiotepa 5 mg/kg days -8, -7, fludara 50 mg/m2 days -6, -5, -4, busulphan 3.2 mg/kg days -6, -5, -4, rabbit ATG 1 mg/Kg day -3, -2). GVHD prophylaxis was with post-transplant cyclophosphamide (50 mg/kg day +3 and +4 at KCH; day +3 and +5 at KFH), mycophenolate and tacrolimus. All patients were in morphological complete remission prior to HSCT. A median 4,8x106 (1,5-14) CD34+/Kg was infused.
Results
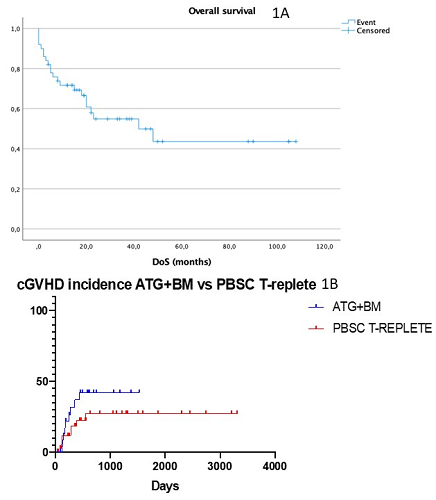
At 24 months 55% of patients were alive and in CR, with median overall survival (OS) not reached (Fig 1A) and no differences between the two cohorts. Two septic deaths before engraftment and 3 primary graft failure were noted. Among 45 evaluable patients, median time to neutrophils ³ 1000/mL was 17 days (13-42), and 25 days (14-44) to platelets ³ 20.000/mL. Median CD3 and CD 15 chimerism at day 100 was 100%. Overall incidence of acute GVHD was 35% (grade I-II 76%; grade III-IV 24%) with no differences between t-replete PBSC and t-deplete BM. cGVHD rate was 26% (30% mild, 13% moderate, 56% severe) and significantly different between the two cohorts (in the PBSC cohort the incidence was 25% vs 40% in the t-deplete BM population (p 0.05 – Fig 1B)). CMV reactivation occurred in 77% of patients, at a median of 42 days after transplant (10-415); 74% patients of KFH had detectable CMV within 4 weeks whereas 54% of KCH’s patients developed CMV reactivation; no cases of CMV disease were noted. The relapse rate at 4 years was 26% for both t-deplete BM and t-replete PBSC (p not significant). Overall, 18 patients (36%) died; cause of death were relapse (57%), GVHD (10%) and sepsis (33%).
Conclusion
this retrospective analysis demonstrated good OS in AML and MDS. The use of a haploidentical protocol with PBSC resulted in a low rate of cGVHD compared to a MA approach that utilised BM with the addition of ATG. Whilst noting the inherent limitations of retrospective data we conclude that PBSC is a safe and effective cell source for haploidentical transplants. Future strategies directed at relapse prevention either via further optimisation of the conditioning, post-HSCT maintenance or cellular therapy will be important for the optimal delivery of the RIC PSBC haplo-HSCT.
Keyword(s): AML, Haploidentical stem cell transplantation, MDS
Abstract: EP1276
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Haploidentical haematopoietic stem cell transplant (haplo-HSCT) is a potentially curative option for patients with myeloid malignancies lacking an HLA identical donor. Although the original platform was based on bone marrow (BM) as cellular source, many transplant centres now use G-CSF mobilised peripheral blood stem cells (PBSC). Chronic graft versus host disease (cGVHD) is a complication that can jeopardize the transplant outcome; for that reason, some centres still prefer BM or use t-depletion with ATG. Relapse rates are also of concern, and the optimal intensity of conditioning (MA vs RIC) in haplo-HSCT for patient with acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) remains unclear.
Aims
The aim of this paper is to compare results of two different haplo-HSCT approaches (BM vs PBSC and MA vs RIC) in patients affected by AML and MDS at King’s College Hospital (KCH) in London and King Faisal Hospital (KFH) in Riyadh.
Methods
Between August 2010 and March 2020, 50 patients with a median age of 46 (14-60yrs) and a high-risk myeloid malignancy were identified (AML n=34, MDS n=16). At KCH, haplo-HSCT (n=23) was performed with G-CSF mobilized PBSC with FluCyTBI protocol (Cy 14.5 mg/kg/ days -6, -5, fludarabine 30 mg/m2/ days -6 to -2, and 200 cGy Total Body Irradiation day -1). At KFH 27 patients had t-deplete BM with TBF regimen (thiotepa 5 mg/kg days -8, -7, fludara 50 mg/m2 days -6, -5, -4, busulphan 3.2 mg/kg days -6, -5, -4, rabbit ATG 1 mg/Kg day -3, -2). GVHD prophylaxis was with post-transplant cyclophosphamide (50 mg/kg day +3 and +4 at KCH; day +3 and +5 at KFH), mycophenolate and tacrolimus. All patients were in morphological complete remission prior to HSCT. A median 4,8x106 (1,5-14) CD34+/Kg was infused.
Results
At 24 months 55% of patients were alive and in CR, with median overall survival (OS) not reached (Fig 1A) and no differences between the two cohorts. Two septic deaths before engraftment and 3 primary graft failure were noted. Among 45 evaluable patients, median time to neutrophils ³ 1000/mL was 17 days (13-42), and 25 days (14-44) to platelets ³ 20.000/mL. Median CD3 and CD 15 chimerism at day 100 was 100%. Overall incidence of acute GVHD was 35% (grade I-II 76%; grade III-IV 24%) with no differences between t-replete PBSC and t-deplete BM. cGVHD rate was 26% (30% mild, 13% moderate, 56% severe) and significantly different between the two cohorts (in the PBSC cohort the incidence was 25% vs 40% in the t-deplete BM population (p 0.05 – Fig 1B)). CMV reactivation occurred in 77% of patients, at a median of 42 days after transplant (10-415); 74% patients of KFH had detectable CMV within 4 weeks whereas 54% of KCH’s patients developed CMV reactivation; no cases of CMV disease were noted. The relapse rate at 4 years was 26% for both t-deplete BM and t-replete PBSC (p not significant). Overall, 18 patients (36%) died; cause of death were relapse (57%), GVHD (10%) and sepsis (33%).

Conclusion
this retrospective analysis demonstrated good OS in AML and MDS. The use of a haploidentical protocol with PBSC resulted in a low rate of cGVHD compared to a MA approach that utilised BM with the addition of ATG. Whilst noting the inherent limitations of retrospective data we conclude that PBSC is a safe and effective cell source for haploidentical transplants. Future strategies directed at relapse prevention either via further optimisation of the conditioning, post-HSCT maintenance or cellular therapy will be important for the optimal delivery of the RIC PSBC haplo-HSCT.
Keyword(s): AML, Haploidentical stem cell transplantation, MDS


