
Contributions
Abstract: EP1273
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Use of mycophenolate mofetil (MMF) has been developed with different prophylactic regimens to obtain effective immunosuppression in combination with calcineurin inhibitors (FK506 or cyclosporine (CsA)) and/or serotherapy (anti-thymocyte immunoglobulin or alemtuzumab). However, the drug presents a significant inter and intra-patient variation that could lead to an inadequate GvHD prophylaxis or, on the contrary, excessive immunosuppression.
Aims
Study of the pharmacokinetics of MMF (MPA AUC) may help to correctly adequate dose and efficacy of GvHD prophylaxis but only a few data are available in pediatric population. Our study aims to find the correct balance in drug dosage to maximize the GvhD prophylaxis without impairing the process of immune recostitution.
Methods
We conducted a retrospective single-centre study at IRCCS “Burlo Garofolo”. The primary goal was to analyze whether FK506 plus MMF prophylactic regimen, with MMF administered dose based on MPA AUC, reduces the incidence of acute and chronic GvHD in pediatric patients comparing with a control historical cohort. Inclusion criteria were age under 18 years, first HSCT, myeloablative conditioning regimen, and at least twelve-month follow-up after HSCT. Control group consisted of CsA and short-course of MTX or CsA and MMF at a standard daily dose of 30 mg/kg with pre-dose plasma level monitoring. All patients in the study group received FK506 and MMF as GvHD prophylaxis. The initial daily dose of MMF in this group was a minimum of 30 mg/kg and subjected to therapeutic drug monitoring (TDM) through MPA AUC.
Results
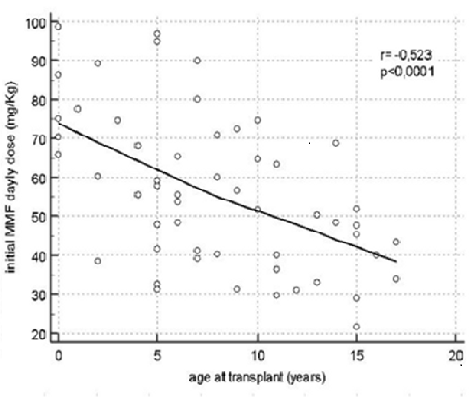
The two populations considered were 55 pts (study group) that received HSCT from 01/11 and 12/19 and 57 patients (control group) from 01/02 to 12/10. The demographics, clinical and HSCT features of the two groups were comparable. (table 1) After a twelve-month follow-up period, we observed a statistically significant difference either in overall acute GvHD (13% vs 69% p<0.0001) and overall chronic GvHD (5% vs 18% p<0.05). Comparing the incidence of different grades of GVHD in the study and the control group, we found statistically significant differences of the rate of acute GvHD I-II grade, acute GvHD III-IV grade, and acute GvHD III-IV grade with donor lymphocyte infusion (DLI)-related onset (45% vs 9%p<0.0001; 22% vs 5% p<0.001, 20% vs 2% p<0.0001, respectively). AUC of MMF leads to change drug dosage one time during the 28 days post-transplant prophylaxis with an increase of dosage mainly under six years of age and a strong inverse correlation (r= -0,523, p<0.0001) between initial daily MMF dose and age at transplant. (figure 1) Finally, no difference was shown in term of relapsed disease in the study group versus the control historical group (20% and 21.3%, respectively).
Conclusion
In our study using a drug monitoring of MMF through the calculation of MPA AUC shows a significant reduction of acute and chronic GvHD incidence. Furthermore, analyzing AUC curves, we saw that patients under six years of age need a significantly higher dose of daily MMF due to greater drug clearance than in older children. Due to the limitation of a retrospective, monocentric study furtherinvestigations are warranted to define efficacy and safety of FK506/MMF prophylaxis.
Keyword(s): Graft-versus-host disease (GVHD)
Abstract: EP1273
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Use of mycophenolate mofetil (MMF) has been developed with different prophylactic regimens to obtain effective immunosuppression in combination with calcineurin inhibitors (FK506 or cyclosporine (CsA)) and/or serotherapy (anti-thymocyte immunoglobulin or alemtuzumab). However, the drug presents a significant inter and intra-patient variation that could lead to an inadequate GvHD prophylaxis or, on the contrary, excessive immunosuppression.
Aims
Study of the pharmacokinetics of MMF (MPA AUC) may help to correctly adequate dose and efficacy of GvHD prophylaxis but only a few data are available in pediatric population. Our study aims to find the correct balance in drug dosage to maximize the GvhD prophylaxis without impairing the process of immune recostitution.
Methods
We conducted a retrospective single-centre study at IRCCS “Burlo Garofolo”. The primary goal was to analyze whether FK506 plus MMF prophylactic regimen, with MMF administered dose based on MPA AUC, reduces the incidence of acute and chronic GvHD in pediatric patients comparing with a control historical cohort. Inclusion criteria were age under 18 years, first HSCT, myeloablative conditioning regimen, and at least twelve-month follow-up after HSCT. Control group consisted of CsA and short-course of MTX or CsA and MMF at a standard daily dose of 30 mg/kg with pre-dose plasma level monitoring. All patients in the study group received FK506 and MMF as GvHD prophylaxis. The initial daily dose of MMF in this group was a minimum of 30 mg/kg and subjected to therapeutic drug monitoring (TDM) through MPA AUC.
Results
The two populations considered were 55 pts (study group) that received HSCT from 01/11 and 12/19 and 57 patients (control group) from 01/02 to 12/10. The demographics, clinical and HSCT features of the two groups were comparable. (table 1) After a twelve-month follow-up period, we observed a statistically significant difference either in overall acute GvHD (13% vs 69% p<0.0001) and overall chronic GvHD (5% vs 18% p<0.05). Comparing the incidence of different grades of GVHD in the study and the control group, we found statistically significant differences of the rate of acute GvHD I-II grade, acute GvHD III-IV grade, and acute GvHD III-IV grade with donor lymphocyte infusion (DLI)-related onset (45% vs 9%p<0.0001; 22% vs 5% p<0.001, 20% vs 2% p<0.0001, respectively). AUC of MMF leads to change drug dosage one time during the 28 days post-transplant prophylaxis with an increase of dosage mainly under six years of age and a strong inverse correlation (r= -0,523, p<0.0001) between initial daily MMF dose and age at transplant. (figure 1) Finally, no difference was shown in term of relapsed disease in the study group versus the control historical group (20% and 21.3%, respectively).

Conclusion
In our study using a drug monitoring of MMF through the calculation of MPA AUC shows a significant reduction of acute and chronic GvHD incidence. Furthermore, analyzing AUC curves, we saw that patients under six years of age need a significantly higher dose of daily MMF due to greater drug clearance than in older children. Due to the limitation of a retrospective, monocentric study furtherinvestigations are warranted to define efficacy and safety of FK506/MMF prophylaxis.
Keyword(s): Graft-versus-host disease (GVHD)


