
Contributions
Abstract: EP1258
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially fatal complication that occurs after hematopoietic cell transplantation (HCT) conditioning or chemotherapy. The most severe form of VOD/SOS is associated with multi-organ dysfunction/failure and a mortality rate of >80% if untreated. Defibrotide is approved in the EU for severe hepatic VOD/SOS post-HCT in patients aged >1 month. Based on the number of patients undergoing HCT reported to the British Society of Bone and Marrow Transplantation (BSBMT) and available commissioned defibrotide use data from National Health Service (NHS) England, the estimated incidence of severe VOD/SOS in patients with first HCT (allogeneic or autologous) in England from 2019-2020 was ~2.5%. Within the NHS Hospital Episode Statistics (HES) database, healthcare resource group (HRG) codes are applied to patient events that have been judged to consume a similar level of resource.
Aims
This analysis used HRG codes to evaluate the incidence of post-HCT VOD/SOS in England and provide details on the effect of HCT and donor type on risk for VOD/SOS.
Methods
In this retrospective analysis, data for patients with first HCT as an inpatient between April 2010 and March 2020 were obtained from the HES database (copyright 2021; reused with the permission of NHS Digital; all rights reserved), a data warehouse containing details of admissions, outpatient appointments, and accident/emergency department attendances at NHS hospitals. Patients were coded for VOD/SOS using ICD-10 diagnostic codes. HRG codes were used to summarize age (≤18 years vs ≥19 years), HCT modality (allogeneic vs autologous) and allogeneic donor type.
Results
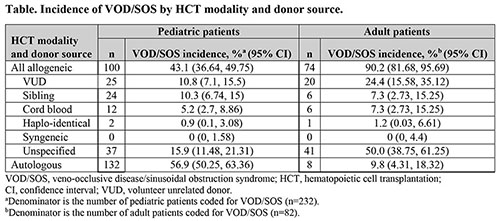
Of the 32,147 identified patients who underwent HCT, 31,387 had evaluable HRG data; 330 (1.05%) of these patients were coded for VOD/SOS (any severity) within 100 days post-HCT. Among the 3,679 pediatric patients (≤18 years) who received HCT, 2,582 (70.2%) received allogeneic HCT and 232 (6.3%) were coded for VOD/SOS. Among the 26,886 adult (≥19 years) patients who received HCT, 8,893 (33.1%) received allogeneic HCT and 82 (0.3%) were coded for VOD/SOS. Age was unknown in 822 patients, 16 of whom were coded for VOD/SOS. VOD/SOS in pediatric patients was primarily reported in the autologous setting (56.9%), in contrast to adults where most VOD/SOS cases were reported in the allogeneic setting (90.2%; Table). There appeared to be a trend to higher VOD/SOS prevalence in groups with increased allogenicity of the donor source but this was not borne out by statistical analyses (Table); among 133 patients with syngeneic HCT, no cases of VOD/SOS were reported.
Conclusion
The incidence of VOD/SOS in this analysis of HRG codes was lower (1.05%) than the ~2.5% incidence rate for severe VOD/SOS obtained from NHS England commissioning data, suggesting that VOD/SOS may be undercoded in the HES database. Limitations of the analysis include restriction to the data available in the HES database, underrecording of haplo-identical HCT in the HES database versus BSBMT returns and the small sample size. In this analysis, there appeared to be higher rates of VOD/SOS in pediatric patients versus adults. VOD/SOS was observed more frequently in the autologous setting in pediatric patients and in the allogeneic setting in adults. These observations suggest that, for allogeneic transplants, there may be a possible relationship between allogenicity of the HCT donor source and incidence of VOD/SOS; further investigation is needed to explore this potential relationship.
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease, VOD
Abstract: EP1258
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially fatal complication that occurs after hematopoietic cell transplantation (HCT) conditioning or chemotherapy. The most severe form of VOD/SOS is associated with multi-organ dysfunction/failure and a mortality rate of >80% if untreated. Defibrotide is approved in the EU for severe hepatic VOD/SOS post-HCT in patients aged >1 month. Based on the number of patients undergoing HCT reported to the British Society of Bone and Marrow Transplantation (BSBMT) and available commissioned defibrotide use data from National Health Service (NHS) England, the estimated incidence of severe VOD/SOS in patients with first HCT (allogeneic or autologous) in England from 2019-2020 was ~2.5%. Within the NHS Hospital Episode Statistics (HES) database, healthcare resource group (HRG) codes are applied to patient events that have been judged to consume a similar level of resource.
Aims
This analysis used HRG codes to evaluate the incidence of post-HCT VOD/SOS in England and provide details on the effect of HCT and donor type on risk for VOD/SOS.
Methods
In this retrospective analysis, data for patients with first HCT as an inpatient between April 2010 and March 2020 were obtained from the HES database (copyright 2021; reused with the permission of NHS Digital; all rights reserved), a data warehouse containing details of admissions, outpatient appointments, and accident/emergency department attendances at NHS hospitals. Patients were coded for VOD/SOS using ICD-10 diagnostic codes. HRG codes were used to summarize age (≤18 years vs ≥19 years), HCT modality (allogeneic vs autologous) and allogeneic donor type.
Results
Of the 32,147 identified patients who underwent HCT, 31,387 had evaluable HRG data; 330 (1.05%) of these patients were coded for VOD/SOS (any severity) within 100 days post-HCT. Among the 3,679 pediatric patients (≤18 years) who received HCT, 2,582 (70.2%) received allogeneic HCT and 232 (6.3%) were coded for VOD/SOS. Among the 26,886 adult (≥19 years) patients who received HCT, 8,893 (33.1%) received allogeneic HCT and 82 (0.3%) were coded for VOD/SOS. Age was unknown in 822 patients, 16 of whom were coded for VOD/SOS. VOD/SOS in pediatric patients was primarily reported in the autologous setting (56.9%), in contrast to adults where most VOD/SOS cases were reported in the allogeneic setting (90.2%; Table). There appeared to be a trend to higher VOD/SOS prevalence in groups with increased allogenicity of the donor source but this was not borne out by statistical analyses (Table); among 133 patients with syngeneic HCT, no cases of VOD/SOS were reported.

Conclusion
The incidence of VOD/SOS in this analysis of HRG codes was lower (1.05%) than the ~2.5% incidence rate for severe VOD/SOS obtained from NHS England commissioning data, suggesting that VOD/SOS may be undercoded in the HES database. Limitations of the analysis include restriction to the data available in the HES database, underrecording of haplo-identical HCT in the HES database versus BSBMT returns and the small sample size. In this analysis, there appeared to be higher rates of VOD/SOS in pediatric patients versus adults. VOD/SOS was observed more frequently in the autologous setting in pediatric patients and in the allogeneic setting in adults. These observations suggest that, for allogeneic transplants, there may be a possible relationship between allogenicity of the HCT donor source and incidence of VOD/SOS; further investigation is needed to explore this potential relationship.
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease, VOD


