Contributions
Abstract: EP1251
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is the standard treatment for relapsed or refractory lymphomas. Until recently, carmustine, etoposide, cytarabine and melphalan (BEAM) was the most used conditioning regimen in this setting, given its acceptable efficacy and tolerability. Recent supply and cost issues for this agent have created an urgent need for alternative conditioning regimens. Toxicity evaluation of alternative conditioning regimens to BEAM may guide the choice of a regimen equally effective and at least equally tolerated, achieving adequate lymphoma control, resulting in similar overall survival.
Aims
To compare toxicities of BEAM (Carmustine, Etoposide, Citarabina, Melphalan), CBV (Carmustine, Etoposide, Cyclophosphamide), BendaEAM (Bendamustine, Etoposide, Citarabina, Melphalan) and BuMel (Busulfan, Melphalan) conditioning regimens used for ASCT in patients with relapsed/refractory non-Hodgkin (NHL) and Hodgkin lymphomas(HL).
Methods
From January 2014 to December 2020, we included patients aged 18 years or older, diagnosed with NHL or HL who underwent ASCT at Hospital das Clinicas da Faculdade de Medicina de Sao Paulo, Brazil. Frequencies of serious toxicities (Grade III-IV CTCAE v4.0) among conditioning schemes were compared using the Fisher's exact test. For the toxicity analysis, a univariate logistic regression model was conducted, and the effect size was presented as odds ratio. Overall survival curves at 100 days and 1 year were determined using the Kaplan Meier method. The assessment of risk factors for mortality was conducted using the multivariate Cox regression method, the effect size on the outcome was presented as a hazard ratio.
Results
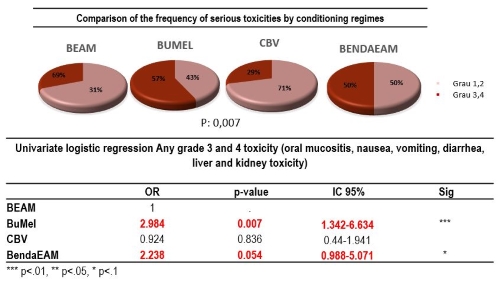
Two hundred and thirteen patients were included (BuMel 42, BendaEAM 38, CBV 65 BEAM 68), of which 56% were men, with a median age of 43 years (IQR 19-74), 93% had a KPS> 80%, and 75% had a complete response prior ASCT. Regarding toxicities grade III and IV: Mucositis was frequently observed in the BuMel regimen 50% and BendaEAM 25%, compared to CBV 10% and BEAM 20%. Diarrhea was present in 30% in the BendaEAM, 28% in the CBV and less frequent in the other groups (BEAM 18% and BuMel 11%); renal toxicity was only presented in the BendaEAM (50%). Febril neutropenia was observed in all the regimens, mainly in the BendaEAM (92%) and BEAM (81%) compared with CBV (76%) and BuMel 67%. In the univariate logistic regression, there was an association of grade III- IV toxicities with type of conditioning regimens (p=0.007) In fact, BuMel (OR 2,984 P0.007) and BendaEAM (OR 2.23 P 0.054) were associated with a greater probability of developing grade III-IV toxicity (Figure 1). Median follow-up of the cohort was 27.51 months (0,75-81,40). Estimated overall survival was 95% at 100 days and 79% at 1 year. The following factors were associated with an increased risk of one year mortality: Ann Arbor stage IV at diagnosis (HR 1.81; P=0.028), disease response at transplant (CR>2) or second partial remission (PR) (HR 3.94; P=0.004) and the use of BendaEAM conditioning (HR 2.86; P=0.05).
Conclusion
BuMel and BendaEAM were associated with higher rate of grade III and IV toxicity however type of conditioning regimen was not ass associated with early mortality Carmustine based conditioning regimens appear to be less toxic and safer. BendaEAM was associated with an increased risk for death at 1 year after autologous transplantation for LNH and LH, but probably related to relapse Longer follow-up is needed to draw definitive conclusions on the use of BendaEAM to control lymphoma relapse.
Keyword(s): Autologous bone marrow transplant, Lymphoma, Stem cell transplant, Toxicity
Abstract: EP1251
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is the standard treatment for relapsed or refractory lymphomas. Until recently, carmustine, etoposide, cytarabine and melphalan (BEAM) was the most used conditioning regimen in this setting, given its acceptable efficacy and tolerability. Recent supply and cost issues for this agent have created an urgent need for alternative conditioning regimens. Toxicity evaluation of alternative conditioning regimens to BEAM may guide the choice of a regimen equally effective and at least equally tolerated, achieving adequate lymphoma control, resulting in similar overall survival.
Aims
To compare toxicities of BEAM (Carmustine, Etoposide, Citarabina, Melphalan), CBV (Carmustine, Etoposide, Cyclophosphamide), BendaEAM (Bendamustine, Etoposide, Citarabina, Melphalan) and BuMel (Busulfan, Melphalan) conditioning regimens used for ASCT in patients with relapsed/refractory non-Hodgkin (NHL) and Hodgkin lymphomas(HL).
Methods
From January 2014 to December 2020, we included patients aged 18 years or older, diagnosed with NHL or HL who underwent ASCT at Hospital das Clinicas da Faculdade de Medicina de Sao Paulo, Brazil. Frequencies of serious toxicities (Grade III-IV CTCAE v4.0) among conditioning schemes were compared using the Fisher's exact test. For the toxicity analysis, a univariate logistic regression model was conducted, and the effect size was presented as odds ratio. Overall survival curves at 100 days and 1 year were determined using the Kaplan Meier method. The assessment of risk factors for mortality was conducted using the multivariate Cox regression method, the effect size on the outcome was presented as a hazard ratio.
Results
Two hundred and thirteen patients were included (BuMel 42, BendaEAM 38, CBV 65 BEAM 68), of which 56% were men, with a median age of 43 years (IQR 19-74), 93% had a KPS> 80%, and 75% had a complete response prior ASCT. Regarding toxicities grade III and IV: Mucositis was frequently observed in the BuMel regimen 50% and BendaEAM 25%, compared to CBV 10% and BEAM 20%. Diarrhea was present in 30% in the BendaEAM, 28% in the CBV and less frequent in the other groups (BEAM 18% and BuMel 11%); renal toxicity was only presented in the BendaEAM (50%). Febril neutropenia was observed in all the regimens, mainly in the BendaEAM (92%) and BEAM (81%) compared with CBV (76%) and BuMel 67%. In the univariate logistic regression, there was an association of grade III- IV toxicities with type of conditioning regimens (p=0.007) In fact, BuMel (OR 2,984 P0.007) and BendaEAM (OR 2.23 P 0.054) were associated with a greater probability of developing grade III-IV toxicity (Figure 1). Median follow-up of the cohort was 27.51 months (0,75-81,40). Estimated overall survival was 95% at 100 days and 79% at 1 year. The following factors were associated with an increased risk of one year mortality: Ann Arbor stage IV at diagnosis (HR 1.81; P=0.028), disease response at transplant (CR>2) or second partial remission (PR) (HR 3.94; P=0.004) and the use of BendaEAM conditioning (HR 2.86; P=0.05).
Conclusion
BuMel and BendaEAM were associated with higher rate of grade III and IV toxicity however type of conditioning regimen was not ass associated with early mortality Carmustine based conditioning regimens appear to be less toxic and safer. BendaEAM was associated with an increased risk for death at 1 year after autologous transplantation for LNH and LH, but probably related to relapse Longer follow-up is needed to draw definitive conclusions on the use of BendaEAM to control lymphoma relapse.
Keyword(s): Autologous bone marrow transplant, Lymphoma, Stem cell transplant, Toxicity