
Contributions
Abstract: EP1238
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Severe hepatic VOD/SOS is a potentially fatal complication of HCT conditioning. Signs and symptoms of VOD/SOS often peak within 14 days of HCT but in a large, prospective study, late-onset (beyond Day 21) VOD/SOS occurred in 26% of patients and was more common in adults than pediatric patients. Defibrotide is approved for severe hepatic VOD/SOS post-HCT in patients aged >1 month in the European Union.
Aims
This subgroup analysis of defibrotide-treated patients from the EBMT PASS evaluated outcomes in patients with VOD/SOS onset ≤21 days or >21 days post-HCT.
Methods
This multicenter, multinational, prospective, observational study (NCT03032016; EBMT PASS), enrolled defibrotide-treated patients from April 2015 to July 2018. Investigators diagnosed VOD/SOS using classical/standard criteria (including, but not limited to, hyperbilirubinemia, hepatomegaly, ascites, and weight gain >5%). Severity grading criteria were not predefined in the protocol; investigators graded VOD/SOS severity based on clinical expertise. Multiorgan dysfunction/failure (MOD/MOF) was diagnosed by the investigator based on renal, pulmonary, or cerebral dysfunction/failure.
Results
Of 104 defibrotide-treated patients with VOD/SOS post-HCT, 82 (79%) had onset ≤21 days (41% were adults) and 22 (21%) had onset >21 days post-HCT (50% were adults). Median time to VOD/SOS onset was 10.5 (range: 1, 21) days in patients with onset ≤21 days and 32.0 (range: 22, 122) days in those with onset >21 days. Median age was 14.0 (range: 0, 69) years and 19.0 (range: 1, 65) years in patients with onset ≤21 days and >21 days, respectively. Severe VOD/SOS occurred in 62 of the 104 patients with VOD/SOS post-HCT; in patients with severe VOD/SOS, 45 (73%) and 17 (27%) patients had VOD/SOS onset ≤21 days and >21 days, respectively. Among patients with severe VOD/SOS, MOD/MOF at baseline was observed in 25 (56%) patients with onset ≤21 days and 11 (65%) patients with onset >21 days.
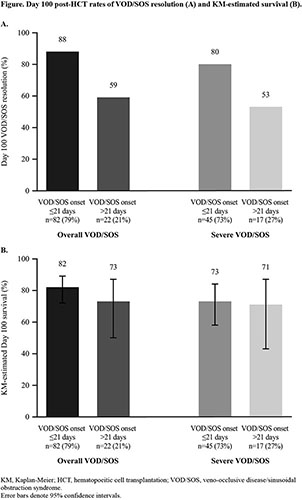
In the overall population, the rates of VOD/SOS resolution (Figure [A]) and Kaplan-Meier–estimated survival (Figure [B]) at Day 100 were numerically lower in patients with late-onset VOD/SOS. Overall, the Kaplan-Meier–estimated survival rate at 1 year post-HCT was 65% in patients with onset ≤21 days and 41% in those with onset >21 days; among patients with severe VOD/SOS, 1-year post-HCT survival rates were 53% and 41%, respectively. In patients with severe VOD/SOS, the MOD/MOF resolution rate was 56% in patients with onset ≤21 days and 46% in those with onset >21 days.
Serious adverse events (SAEs) of interest occurred in 29% of patients with onset ≤21 days and 23% of those with onset >21 days; among patients with severe VOD/SOS, incidence of SAEs was 33% and 29%, respectively. The most common categories of SAEs in the overall population were infections (onset ≤21 days: 22%; onset >21 days: 14%) and bleeding events (onset ≤21 days: 11%; onset >21 days: 14%); corresponding values among patients with severe VOD/SOS were 27% and 18% for infections and 11% and 18% for bleeding events.
Conclusion
In this analysis of post-HCT VOD/SOS patients, approximately 20% developed VOD/SOS >21 days post-HCT, highlighting the need for continued vigilance for VOD/SOS beyond the usual window of development. Given that patients with late-onset VOD/SOS are likely to begin treatment later post-HCT than those with onset ≤21 days, caution must be taken when interpreting rates of Day 100 post-HCT VOD/SOS resolution and survival. The safety of defibrotide in this real-world setting was consistent with previous studies.
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease, VOD
Abstract: EP1238
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Severe hepatic VOD/SOS is a potentially fatal complication of HCT conditioning. Signs and symptoms of VOD/SOS often peak within 14 days of HCT but in a large, prospective study, late-onset (beyond Day 21) VOD/SOS occurred in 26% of patients and was more common in adults than pediatric patients. Defibrotide is approved for severe hepatic VOD/SOS post-HCT in patients aged >1 month in the European Union.
Aims
This subgroup analysis of defibrotide-treated patients from the EBMT PASS evaluated outcomes in patients with VOD/SOS onset ≤21 days or >21 days post-HCT.
Methods
This multicenter, multinational, prospective, observational study (NCT03032016; EBMT PASS), enrolled defibrotide-treated patients from April 2015 to July 2018. Investigators diagnosed VOD/SOS using classical/standard criteria (including, but not limited to, hyperbilirubinemia, hepatomegaly, ascites, and weight gain >5%). Severity grading criteria were not predefined in the protocol; investigators graded VOD/SOS severity based on clinical expertise. Multiorgan dysfunction/failure (MOD/MOF) was diagnosed by the investigator based on renal, pulmonary, or cerebral dysfunction/failure.
Results
Of 104 defibrotide-treated patients with VOD/SOS post-HCT, 82 (79%) had onset ≤21 days (41% were adults) and 22 (21%) had onset >21 days post-HCT (50% were adults). Median time to VOD/SOS onset was 10.5 (range: 1, 21) days in patients with onset ≤21 days and 32.0 (range: 22, 122) days in those with onset >21 days. Median age was 14.0 (range: 0, 69) years and 19.0 (range: 1, 65) years in patients with onset ≤21 days and >21 days, respectively. Severe VOD/SOS occurred in 62 of the 104 patients with VOD/SOS post-HCT; in patients with severe VOD/SOS, 45 (73%) and 17 (27%) patients had VOD/SOS onset ≤21 days and >21 days, respectively. Among patients with severe VOD/SOS, MOD/MOF at baseline was observed in 25 (56%) patients with onset ≤21 days and 11 (65%) patients with onset >21 days.
In the overall population, the rates of VOD/SOS resolution (Figure [A]) and Kaplan-Meier–estimated survival (Figure [B]) at Day 100 were numerically lower in patients with late-onset VOD/SOS. Overall, the Kaplan-Meier–estimated survival rate at 1 year post-HCT was 65% in patients with onset ≤21 days and 41% in those with onset >21 days; among patients with severe VOD/SOS, 1-year post-HCT survival rates were 53% and 41%, respectively. In patients with severe VOD/SOS, the MOD/MOF resolution rate was 56% in patients with onset ≤21 days and 46% in those with onset >21 days.
Serious adverse events (SAEs) of interest occurred in 29% of patients with onset ≤21 days and 23% of those with onset >21 days; among patients with severe VOD/SOS, incidence of SAEs was 33% and 29%, respectively. The most common categories of SAEs in the overall population were infections (onset ≤21 days: 22%; onset >21 days: 14%) and bleeding events (onset ≤21 days: 11%; onset >21 days: 14%); corresponding values among patients with severe VOD/SOS were 27% and 18% for infections and 11% and 18% for bleeding events.

Conclusion
In this analysis of post-HCT VOD/SOS patients, approximately 20% developed VOD/SOS >21 days post-HCT, highlighting the need for continued vigilance for VOD/SOS beyond the usual window of development. Given that patients with late-onset VOD/SOS are likely to begin treatment later post-HCT than those with onset ≤21 days, caution must be taken when interpreting rates of Day 100 post-HCT VOD/SOS resolution and survival. The safety of defibrotide in this real-world setting was consistent with previous studies.
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease, VOD


