
Contributions
Abstract: EP1235
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Improvements in supportive care and HCT practices have led to increasing use of HDT-AHCT for patients with chemosensitive lymphomas, with a growing interest of treating older patients (pts). However, HDT is associated with high rates of severe regimen-related toxicities (SRRT), especially in older adults, (Dahi 2018) and the risk of life-threatening SRRT may preclude older pts or pts with co-morbidities from this potentially curative treatment.
SRRT is thought to result from diffuse injury to the microvasculature forming the organ vascular stem cell niches caused by off-target cytotoxic effects of HDT. SRRT involves virtually all organ systems, but is most evident in organs with high cell turnover such as oral-gastrointestinal (oral/GI) tract and bone marrow. While the hematopoietic system is rescued by AHCT, there are no effective therapies to repair damage to other organs.
AB-205 is a novel experimental engineered-cell therapy. AB-205 contains E-CEL® cells: allogeneic E4+ human cord endothelial cells. E-CEL cells express multiple angiocrine factors in a dynamic, paracrine manner.
Aims
AB-205-001 objectives included assessing initial safety and efficacy.
Methods
Subjects with chemosensitive lymphomas eligible for HDT-AHCT were enrolled. AB-205 was administered IV after AHCT in dose-escalated cohorts: 5, 10 and 20x106 cells/kg, either as single or divided (D0, D2) dose. Supportive care therapies were administered as per site institutional guidelines. Oral/GI SRRT was defined as NCI-CTCAEv5.0 grade (G)≥3 oral mucositis, nausea, vomiting or diarrhea. A retrospective chart review (n=45) at a participating site served as a contemporary control.
Results
Overall results have been presented previously [ASH 2020, TCT 2021, EBMT 2021]. Here we present data from pts treated with 20x106 cells/kg by age.
29 systemic lymphoma subjects were treated with AB-205 with a median (range) follow up of 271 (179, 566) days. MTD was not reached. AEs were generally mild/moderate and as expected with HDT-AHCT.
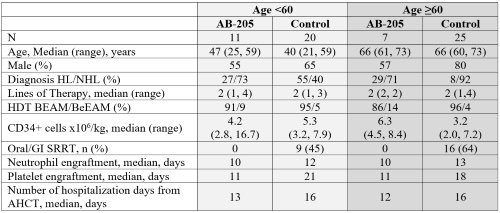
AB-205 therapy demonstrated dose-dependent reduction of oral/GI SRRT. No (0%) oral/GI SRRT were reported for any subjects (n=18) receiving the recommended AB-205 dose, including the subjects ≥60 yo (n=7). In contrast, the control cohort reported 16/25 (64%) oral/GI SRRT rate ≥60 yo, while those <60 yo had 9/20 (45%).
Median time to neutrophil engraftment, platelet engraftment and number of hospitalization days (from AHCT) were numerically lower with AB-205 than in the control cohort by approximately 2, 7 and 3 days respectively (Table). The benefits with AB-205 treatment were consistent across age subgroups.
As of the cutoff date, there have been no reports of SRRT affecting the pulmonary, cardiac, renal or hepatic systems. No detrimental effect on PFS or OS was observed. There were no deaths by day 100.
Conclusion
20x106 cells/kg of AB-205 eliminated the occurrence of oral/GI SRRT including in subjects 60 yo. In contrast, 64% event rates were seen in pts ≥60 yo in a contemporary control cohort. AB-205 has potential to make HDT-AHCT substantially safer for older pts and may enable physicians to consider this potentially curative therapy for high-risk patients currently considered ineligible for HCT.
Keyword(s): Autologous hematopoietic stem cell transplantation, Cellular therapy, Lymphoma therapy, Supportive care
Abstract: EP1235
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Improvements in supportive care and HCT practices have led to increasing use of HDT-AHCT for patients with chemosensitive lymphomas, with a growing interest of treating older patients (pts). However, HDT is associated with high rates of severe regimen-related toxicities (SRRT), especially in older adults, (Dahi 2018) and the risk of life-threatening SRRT may preclude older pts or pts with co-morbidities from this potentially curative treatment.
SRRT is thought to result from diffuse injury to the microvasculature forming the organ vascular stem cell niches caused by off-target cytotoxic effects of HDT. SRRT involves virtually all organ systems, but is most evident in organs with high cell turnover such as oral-gastrointestinal (oral/GI) tract and bone marrow. While the hematopoietic system is rescued by AHCT, there are no effective therapies to repair damage to other organs.
AB-205 is a novel experimental engineered-cell therapy. AB-205 contains E-CEL® cells: allogeneic E4+ human cord endothelial cells. E-CEL cells express multiple angiocrine factors in a dynamic, paracrine manner.
Aims
AB-205-001 objectives included assessing initial safety and efficacy.
Methods
Subjects with chemosensitive lymphomas eligible for HDT-AHCT were enrolled. AB-205 was administered IV after AHCT in dose-escalated cohorts: 5, 10 and 20x106 cells/kg, either as single or divided (D0, D2) dose. Supportive care therapies were administered as per site institutional guidelines. Oral/GI SRRT was defined as NCI-CTCAEv5.0 grade (G)≥3 oral mucositis, nausea, vomiting or diarrhea. A retrospective chart review (n=45) at a participating site served as a contemporary control.
Results
Overall results have been presented previously [ASH 2020, TCT 2021, EBMT 2021]. Here we present data from pts treated with 20x106 cells/kg by age.
29 systemic lymphoma subjects were treated with AB-205 with a median (range) follow up of 271 (179, 566) days. MTD was not reached. AEs were generally mild/moderate and as expected with HDT-AHCT.
AB-205 therapy demonstrated dose-dependent reduction of oral/GI SRRT. No (0%) oral/GI SRRT were reported for any subjects (n=18) receiving the recommended AB-205 dose, including the subjects ≥60 yo (n=7). In contrast, the control cohort reported 16/25 (64%) oral/GI SRRT rate ≥60 yo, while those <60 yo had 9/20 (45%).
Median time to neutrophil engraftment, platelet engraftment and number of hospitalization days (from AHCT) were numerically lower with AB-205 than in the control cohort by approximately 2, 7 and 3 days respectively (Table). The benefits with AB-205 treatment were consistent across age subgroups.
As of the cutoff date, there have been no reports of SRRT affecting the pulmonary, cardiac, renal or hepatic systems. No detrimental effect on PFS or OS was observed. There were no deaths by day 100.

Conclusion
20x106 cells/kg of AB-205 eliminated the occurrence of oral/GI SRRT including in subjects 60 yo. In contrast, 64% event rates were seen in pts ≥60 yo in a contemporary control cohort. AB-205 has potential to make HDT-AHCT substantially safer for older pts and may enable physicians to consider this potentially curative therapy for high-risk patients currently considered ineligible for HCT.
Keyword(s): Autologous hematopoietic stem cell transplantation, Cellular therapy, Lymphoma therapy, Supportive care


