Contributions
Abstract: EP1228
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Allogeneic hematopoietic cell transplantation (allo-HCT) in patients with Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) in first complete remission (CR1) has improved survival outcomes, even after the advent of tyrosine kinase inhibitors (TKI). However, relapses post allo-HCT still occur in up to 30% of transplanted patients, and pose a real challenge. While historically, available treatment options involved reduction/withdrawal of immunosuppression, cytotoxic chemotherapy, donor lymphocyte infusion, and a second allo-HCT, currently there is an increased use of newer generation TKI, monoclonal antibodies such blinatumomab and inotuzumab, as well as CAR T cell therapy.
Aims
The aim of this study was to assess the impact of the post-transplant relapse therapeutic evolution on survival outcomes over the years.
Methods
We selected all adult patients from the EBMT database with a diagnosis of Ph+ ALL who had morphologic relapse between 2000 and 2019 after allo-HCT performed in CR1, regardless of type of conditioning, donor type, stem cell source and prior TKI treatment. We analyzed survival outcomes using the Kaplan Meier method, and predictive factors by univariate and multivariate analysis. Median follow up for alive patients was 56 months.
Results
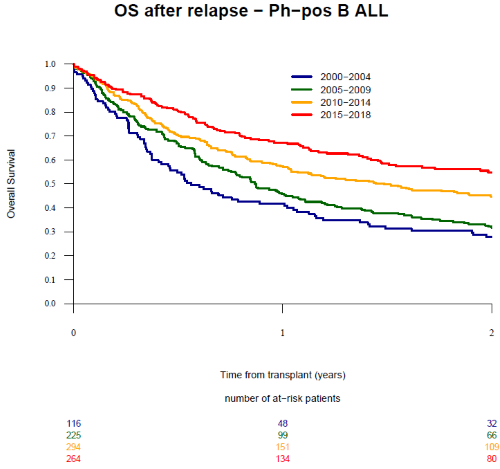
We identified 899 patients with a median age at transplant and at relapse of 44 and 45.4 years, respectively. Overall, 116 patients relapsed between 2000-2004, 225 between 2005-2009, 294 between 2010-2014, and 264 between 2015-2019. Patient and transplant characteristics were similar over the 4 time periods except for a progressive increase in the use of matched unrelated donor (from 34.5% between 2000-2004 to 55.7% between 2015-2019; p=0.0002), peripheral blood stem cells (from 60.3% to 84.5%; p<0.0001), reduced intensity conditioning (RIC) (from 16.4% to 34.5%; p=0.004), and in vivo T cell depletion (TCD) (from 27.9% to 62.4%; p=0.0001) as well as a progressive decrease in the use of total body irradiation (TBI) (from 73.3% to 53%; p=0.0002), respectively. The 2-year overall survival (OS) after relapse was 41.5 % (95% CI: 38 - 44.9). Original disease was the cause of death in 68.5% of patients. Importantly, the 2-year OS after relapse increased from 27.8% for patients relapsing between 2000-2004 to 31.7% for 2005-2009, 44.5% for 2010-2014 and 54.8% for 2015-2019 (p=0.001) (Figure 1). A second allo-HCT within 2 years after relapse was performed in 13.9% of patients resulting in a 2-year OS of 35.9%. The incidence of second allo-HCT was 21.7%, 12.8%, 9.9% and 15.7% for the same time periods (p=0.027). In multivariate analysis, factors that influenced positively the OS were a longer time from transplant to relapse (>median:7.1 months; hazard ratio [HR] 0.71, p=0.0007) and the year of relapse (reference 2000-2004; HR 0.68, p<0.013 for patients relapsing from 2005-2009; HR 0.72; p<0.008 for patients relapsing from 2010-2014, and HR=0.72; p=0.016 for patients relapsing from 2015-2019). Other patient, donor and transplant characteristics had no significant effect on outcomes.
Conclusion
We observe a major progressive improvement in OS from post-transplant relapse for patients with Ph+ ALL, likely reflecting the efficacy of post-transplant therapeutic strategies. This large-scale real-world data can serve as a reference for future studies investigating novel agents in this setting.
Keyword(s):
Abstract: EP1228
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Allogeneic hematopoietic cell transplantation (allo-HCT) in patients with Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) in first complete remission (CR1) has improved survival outcomes, even after the advent of tyrosine kinase inhibitors (TKI). However, relapses post allo-HCT still occur in up to 30% of transplanted patients, and pose a real challenge. While historically, available treatment options involved reduction/withdrawal of immunosuppression, cytotoxic chemotherapy, donor lymphocyte infusion, and a second allo-HCT, currently there is an increased use of newer generation TKI, monoclonal antibodies such blinatumomab and inotuzumab, as well as CAR T cell therapy.
Aims
The aim of this study was to assess the impact of the post-transplant relapse therapeutic evolution on survival outcomes over the years.
Methods
We selected all adult patients from the EBMT database with a diagnosis of Ph+ ALL who had morphologic relapse between 2000 and 2019 after allo-HCT performed in CR1, regardless of type of conditioning, donor type, stem cell source and prior TKI treatment. We analyzed survival outcomes using the Kaplan Meier method, and predictive factors by univariate and multivariate analysis. Median follow up for alive patients was 56 months.
Results
We identified 899 patients with a median age at transplant and at relapse of 44 and 45.4 years, respectively. Overall, 116 patients relapsed between 2000-2004, 225 between 2005-2009, 294 between 2010-2014, and 264 between 2015-2019. Patient and transplant characteristics were similar over the 4 time periods except for a progressive increase in the use of matched unrelated donor (from 34.5% between 2000-2004 to 55.7% between 2015-2019; p=0.0002), peripheral blood stem cells (from 60.3% to 84.5%; p<0.0001), reduced intensity conditioning (RIC) (from 16.4% to 34.5%; p=0.004), and in vivo T cell depletion (TCD) (from 27.9% to 62.4%; p=0.0001) as well as a progressive decrease in the use of total body irradiation (TBI) (from 73.3% to 53%; p=0.0002), respectively. The 2-year overall survival (OS) after relapse was 41.5 % (95% CI: 38 - 44.9). Original disease was the cause of death in 68.5% of patients. Importantly, the 2-year OS after relapse increased from 27.8% for patients relapsing between 2000-2004 to 31.7% for 2005-2009, 44.5% for 2010-2014 and 54.8% for 2015-2019 (p=0.001) (Figure 1). A second allo-HCT within 2 years after relapse was performed in 13.9% of patients resulting in a 2-year OS of 35.9%. The incidence of second allo-HCT was 21.7%, 12.8%, 9.9% and 15.7% for the same time periods (p=0.027). In multivariate analysis, factors that influenced positively the OS were a longer time from transplant to relapse (>median:7.1 months; hazard ratio [HR] 0.71, p=0.0007) and the year of relapse (reference 2000-2004; HR 0.68, p<0.013 for patients relapsing from 2005-2009; HR 0.72; p<0.008 for patients relapsing from 2010-2014, and HR=0.72; p=0.016 for patients relapsing from 2015-2019). Other patient, donor and transplant characteristics had no significant effect on outcomes.
Conclusion
We observe a major progressive improvement in OS from post-transplant relapse for patients with Ph+ ALL, likely reflecting the efficacy of post-transplant therapeutic strategies. This large-scale real-world data can serve as a reference for future studies investigating novel agents in this setting.
Keyword(s):