
Contributions
Abstract: EP1226
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
The prognosis of 11q23/KMT2A rearranged (KMT2A-r) acute leukemia (AL) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) was poor. Minimal residual disease (MRD) was an important prognostic factor for relapse.MRD directed preemptive immunotherapy was effective to prevent relapse after allo-HSCT in AL. However, no study had identified the efficacy of these methods in a disease-specific population of patients with KMT2A-r AL, and whether commonly used preemptive immunotherapies could clear the MRD in these patients was unknown.
Aims
We aimed to identify the evolution of KMT2A before and after allo-HSCT and the efficacy of preemptive immunotherapies for KMT2A-r AL patients receiving allo-HSCT.
Methods
KMT2A expression was determined through TaqMan-based RQ-PCR technology. Preemptive immunotherapies included interferon-α and donor lymphocyte infusion. We collected 1751 bone marrow samples from 177 consecutive KMT2A-r AL patients.The SPSS 23 and the R software package were used for data analyses.
Results
Pre-HSCT KMT2A positive was correlated with post-HSCT KMT2A positive (correlation coefficient=0.371, P<0.001). The rates of KMT2A achieving negative after allo-HSCT were 96.6%, 92.9%, and 68.8% in the pre-HSCT low (>0, <0.1%)-, intermediate (≥ 0.1%, <1%)-, and high-level (≥1%) groups, respectively. The rates of regaining KMT2A positive after allo-HSCT were 7.7%, 35.7%, 38.5%, and 45.5%, respectively, for pre-HSCT KMT2A negative, low-, intermediate-, and high-level groups (P<0.001). The 4-year cumulative incidence of relapse after allo-HSCT was as high as 53.7% in pre-HSCT KMT2A expression ≥ 0.1% group, which was comparable between the KMT2A negative (15.1%) and KMT2A <0.1% group (31.2%). The clinical outcomes of patients with post-HSCT KMT2A positive were poorer than those with persistent KMT2A negative.
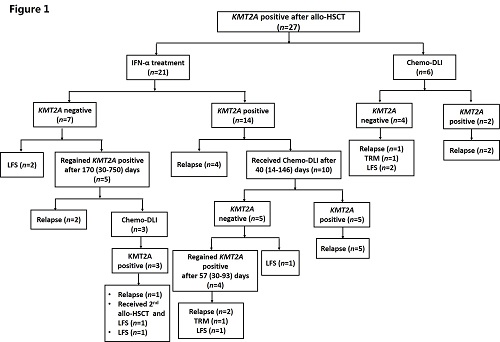
Thirty patients showed KMT2A positive after allo-HSCT, and the detail of KMT2A-directed immunotherapies were showed in Figure 1. In total, 27 KMT2A patients (25 AML and 2 ALL) received preemptive immunotherapies. Twenty-one patients received preemptive IFN-α treatment. Seven patients (33.3%) achieved KMT2A negative; however, 5 of them regained KMT2A positive, and only 2 patients (9.5%) achieved persistent LFS after IFN-α treatment. Nineteen patients received preemptive Chemo-DLI. Nine patients (47.4%) achieved KMT2A negative. In addition, only the patients with very low-level KMT2A gene expression might benefit from this treatment (rate of KMT2A achieving negative: KMT2A <0.01% vs. ≥ 0.01% before IFN-α treatment, 75.0% vs. 23.5%, P=0.088). Thirteen patients (68.4%) experienced relapsed and 5 patients (26.3%) achieved persistent LFS. The level of KMT2A gene expression before Chemo-DLI did not influence the rate of KMT2A achieving negative (<0.1% vs. ≥ 0.1% before Chemo-DLI, 75.0% vs. 46.2%, P=0.576).
Conclusion
Pre-HSCT KMT2A positive were significantly associated with post-HSCT KMT2A positive. The clinical outcomes of patients with post-HSCT KMT2A positive was poor. Although post-HSCT preemptive immunotherapies might help to achieve KMT2A negative, the long-term efficacy was unsatisfactory.
Keyword(s): 11q23, Acute leukemia, Allogeneic hematopoietic stem cell transplant, Minimal residual disease (MRD)
Abstract: EP1226
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
The prognosis of 11q23/KMT2A rearranged (KMT2A-r) acute leukemia (AL) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) was poor. Minimal residual disease (MRD) was an important prognostic factor for relapse.MRD directed preemptive immunotherapy was effective to prevent relapse after allo-HSCT in AL. However, no study had identified the efficacy of these methods in a disease-specific population of patients with KMT2A-r AL, and whether commonly used preemptive immunotherapies could clear the MRD in these patients was unknown.
Aims
We aimed to identify the evolution of KMT2A before and after allo-HSCT and the efficacy of preemptive immunotherapies for KMT2A-r AL patients receiving allo-HSCT.
Methods
KMT2A expression was determined through TaqMan-based RQ-PCR technology. Preemptive immunotherapies included interferon-α and donor lymphocyte infusion. We collected 1751 bone marrow samples from 177 consecutive KMT2A-r AL patients.The SPSS 23 and the R software package were used for data analyses.
Results
Pre-HSCT KMT2A positive was correlated with post-HSCT KMT2A positive (correlation coefficient=0.371, P<0.001). The rates of KMT2A achieving negative after allo-HSCT were 96.6%, 92.9%, and 68.8% in the pre-HSCT low (>0, <0.1%)-, intermediate (≥ 0.1%, <1%)-, and high-level (≥1%) groups, respectively. The rates of regaining KMT2A positive after allo-HSCT were 7.7%, 35.7%, 38.5%, and 45.5%, respectively, for pre-HSCT KMT2A negative, low-, intermediate-, and high-level groups (P<0.001). The 4-year cumulative incidence of relapse after allo-HSCT was as high as 53.7% in pre-HSCT KMT2A expression ≥ 0.1% group, which was comparable between the KMT2A negative (15.1%) and KMT2A <0.1% group (31.2%). The clinical outcomes of patients with post-HSCT KMT2A positive were poorer than those with persistent KMT2A negative.
Thirty patients showed KMT2A positive after allo-HSCT, and the detail of KMT2A-directed immunotherapies were showed in Figure 1. In total, 27 KMT2A patients (25 AML and 2 ALL) received preemptive immunotherapies. Twenty-one patients received preemptive IFN-α treatment. Seven patients (33.3%) achieved KMT2A negative; however, 5 of them regained KMT2A positive, and only 2 patients (9.5%) achieved persistent LFS after IFN-α treatment. Nineteen patients received preemptive Chemo-DLI. Nine patients (47.4%) achieved KMT2A negative. In addition, only the patients with very low-level KMT2A gene expression might benefit from this treatment (rate of KMT2A achieving negative: KMT2A <0.01% vs. ≥ 0.01% before IFN-α treatment, 75.0% vs. 23.5%, P=0.088). Thirteen patients (68.4%) experienced relapsed and 5 patients (26.3%) achieved persistent LFS. The level of KMT2A gene expression before Chemo-DLI did not influence the rate of KMT2A achieving negative (<0.1% vs. ≥ 0.1% before Chemo-DLI, 75.0% vs. 46.2%, P=0.576).

Conclusion
Pre-HSCT KMT2A positive were significantly associated with post-HSCT KMT2A positive. The clinical outcomes of patients with post-HSCT KMT2A positive was poor. Although post-HSCT preemptive immunotherapies might help to achieve KMT2A negative, the long-term efficacy was unsatisfactory.
Keyword(s): 11q23, Acute leukemia, Allogeneic hematopoietic stem cell transplant, Minimal residual disease (MRD)


