
Contributions
Abstract: EP1225
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Donor-specific anti-HLA antibodies (DSAs) are antibodies in the recipient directed against donor class I/II HLA antigens. The existence of DSAs before HLA mismatched allogenic hematopoietic stem cell transplantation (allo-HCT) are known to cause primary graft failure. Currently, there is no established method of desensitization for DSAs due to the long half-life of plasma cells.
Aims
To evaluate the toxicity and efficacy of the BCMA-CD19 cCAR in the reduction of Donor-Specific Antibody (DSA) in haploidentical hematopoietic stem cell transplantation(haplo-HCT).
Methods
We have generated a BCMA-CD19 cCAR bearing 2 complete CAR constructs connected by a cleavable linker, P2A. CAR T cells were manufactured in a cGMP facility. The in vitro and in vivo activity of the cCAR were detected by co-culture assays and in mouse model studies. Between May 2019 and Sep 2020, 4 haploidentical HCT candidates with high titers of DSA were enrolled. All patients received conditioning (fludarabine 30 mg/m2/d and cyclophosphamide 300 mg/m2/d for 3 days). CAR-T cells were given by a dose escalation at 2~4.5×106/kg with a single dose. Study endpoints include the safety of the CAR, the reduction of DSA and engraftment after haplo-HCT.
Results
In co-culture assays, BCMA-CD19 CAR-T cells exhibited robust cytotoxic activity against the K562 cells engineered to express either CD19 or BCMA. In mouse model studies, cCAR-T cells were able to eliminate tumor in mice injected with MM.1S cells and REH cells, indicating the ability of the cCAR to specifically lyse both BCMA and CD19 expressing target cells in vitro and in vivo.
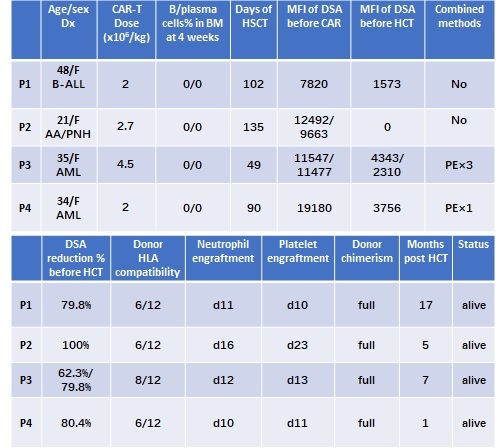
Of the 4 patients who have received CAR-T cell infusion, 1 was acute lymphoblastic leukemia(ALL), 2 were acute myeloid leukemia(AML), 1 was aplastic anemia-paroxysmal nocturnal hemoglobinuria (AA-PNH) syndrome. The age was from 21 to 48 years old, all patients had high DSA levels (MFI >5,000). CAR-T dose were from 2-4.5×106/kg.
Cytokine release syndrome (CRS) occurred in 3 patients (1 grade Ⅰ, 2 grade Ⅱ). No Neurotoxicity and other severe organ toxicity occurred in all the patients.
Bone marrow B cells and plasma cells were depleted at 4 weeks post CAR-T therapy in all patients. DSA dropped to low levels (MFI <5,000) for all patients, of them, plasma exchange was combined in 2 patients due to the emergency of HCT. The reduction percent ranged from 62.3% to 100%. All patients received a haplo-HCT 49-135 days post CAR-T therapy, and were successfully engrafted with a persistent full chimerism. (Figure 1)
Conclusion
Our first in human clinical trial on BCMA-CD19 cCAR demonstrated profound efficacy in reducing DSA levels in an allo-HCT candidate. There was strong clinical evidence of depletion of antibody-producing roots, B-cells and plasma cells in the patients. Our results further suggested that BCMA-CD19 cCAR has the potential to benefit patients receiving solid organ transplants or those with other antibody-mediated diseases.
Keyword(s): B-cell maturation antigen, BMT, CAR-T, CD19
Abstract: EP1225
Type: E-Poster Presentation
Session title: Stem cell transplantation - Clinical
Background
Donor-specific anti-HLA antibodies (DSAs) are antibodies in the recipient directed against donor class I/II HLA antigens. The existence of DSAs before HLA mismatched allogenic hematopoietic stem cell transplantation (allo-HCT) are known to cause primary graft failure. Currently, there is no established method of desensitization for DSAs due to the long half-life of plasma cells.
Aims
To evaluate the toxicity and efficacy of the BCMA-CD19 cCAR in the reduction of Donor-Specific Antibody (DSA) in haploidentical hematopoietic stem cell transplantation(haplo-HCT).
Methods
We have generated a BCMA-CD19 cCAR bearing 2 complete CAR constructs connected by a cleavable linker, P2A. CAR T cells were manufactured in a cGMP facility. The in vitro and in vivo activity of the cCAR were detected by co-culture assays and in mouse model studies. Between May 2019 and Sep 2020, 4 haploidentical HCT candidates with high titers of DSA were enrolled. All patients received conditioning (fludarabine 30 mg/m2/d and cyclophosphamide 300 mg/m2/d for 3 days). CAR-T cells were given by a dose escalation at 2~4.5×106/kg with a single dose. Study endpoints include the safety of the CAR, the reduction of DSA and engraftment after haplo-HCT.
Results
In co-culture assays, BCMA-CD19 CAR-T cells exhibited robust cytotoxic activity against the K562 cells engineered to express either CD19 or BCMA. In mouse model studies, cCAR-T cells were able to eliminate tumor in mice injected with MM.1S cells and REH cells, indicating the ability of the cCAR to specifically lyse both BCMA and CD19 expressing target cells in vitro and in vivo.
Of the 4 patients who have received CAR-T cell infusion, 1 was acute lymphoblastic leukemia(ALL), 2 were acute myeloid leukemia(AML), 1 was aplastic anemia-paroxysmal nocturnal hemoglobinuria (AA-PNH) syndrome. The age was from 21 to 48 years old, all patients had high DSA levels (MFI >5,000). CAR-T dose were from 2-4.5×106/kg.
Cytokine release syndrome (CRS) occurred in 3 patients (1 grade Ⅰ, 2 grade Ⅱ). No Neurotoxicity and other severe organ toxicity occurred in all the patients.
Bone marrow B cells and plasma cells were depleted at 4 weeks post CAR-T therapy in all patients. DSA dropped to low levels (MFI <5,000) for all patients, of them, plasma exchange was combined in 2 patients due to the emergency of HCT. The reduction percent ranged from 62.3% to 100%. All patients received a haplo-HCT 49-135 days post CAR-T therapy, and were successfully engrafted with a persistent full chimerism. (Figure 1)

Conclusion
Our first in human clinical trial on BCMA-CD19 cCAR demonstrated profound efficacy in reducing DSA levels in an allo-HCT candidate. There was strong clinical evidence of depletion of antibody-producing roots, B-cells and plasma cells in the patients. Our results further suggested that BCMA-CD19 cCAR has the potential to benefit patients receiving solid organ transplants or those with other antibody-mediated diseases.
Keyword(s): B-cell maturation antigen, BMT, CAR-T, CD19


