
Contributions
Abstract: EP1208
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
The Belgian sickle cell disease registry (BSR) was initiated in 2008 and updated in 2018 to be a more user-friendly and easy-operable application.
Aims
To evaluate if disease modifying treatment (DMT) practice across centres participating in BSR is similar and what the mortality rate is among patients with sickle cell disease (SCD) included in the BSR.
Methods
All data from the initial 2008 database cohort were retrospectively encoded in the updated database format. All new data are recorded prospectively from neonatal screening or first contact until last annual follow-up (FU) or death. The data collected included diagnosis, demography, treatment and outcome data. Data were extracted from the database in January 2021 and were registered by 13 different centres.
Results
There were 977 patients registered with a median FU of 9 years (1-53 y). Median age at last FU was 13 (0-60 y). 654 (87,5%) have a severe phenotype (SS or Sβ°) and 51% are female. Among them, 546 (56%) were born in Belgium of which 369 (68%) were diagnosed by neonatal screening. In the absence of a neonatal screening, median age at diagnosis was 1 year (range 0-18). At last follow-up, 707 patients (72.3%) receive DMT: 523 patients receive hydroxyurea (HU), 126 underwent hematopoietic stem cell transplantation (HSCT), 53 are chronically transfused, 5 participate to a study with crizanlizumab.
Among not transplanted patients, the proportion of those receiving HU is significantly different among centres (46% to 75% ; p<0,0001). The median age at which HU was initiated was also significantly different among the centres (4y to 21y; p<0,0001).
Most of HSCT were performed in two main centres i.e., 66 and 58, respectively. Median age at HSCT was significantly different between both centres ( 8y (2-15) versus 5y (0-19) ; p=0,004).
Twenty-four (2.5%) patients died which account for a mortality rate of 0.22/100 patients-years (PY) and a trend to increase with age (0,18/100PY < 18 years, 0.39/100PY 18 -40 years and 0.63/100PY > 40 years ; p=0.056) .
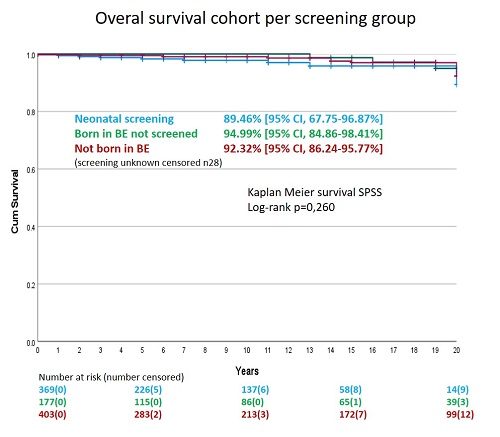
Kaplan-Meier estimate of survival at 20 years is similar regardless of the mode of diagnosis (89%, 95%, 92% respectively for neonatal screening, not screened and not born in Belgium; p=0.260)(Figure 1).
Conclusion
Among the patient cohort recorded in the BSR, the proportion of patients receiving DMT, the age at start of HU and the age at HSCT is significantly different between centres. A detailed analysis of practices based at least on demographic criteria, severity of the disease, etc. is needed to understand their causes. Mortality remains low but with a trend to increase with age. The absence of a national neonatal screening program, deaths occurring before diagnosis and no inclusion in the registry, represent a bias in the interpretation of the mortality. Survival status might be impacted by the proportion of patients that consented to participate to the BSR and could be different between the neonatal screening, born in Belgium not screened, and born abroad groups.
Keyword(s): HSCT, Hydroxyurea, Mortality, Sickle cell disease
Abstract: EP1208
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
The Belgian sickle cell disease registry (BSR) was initiated in 2008 and updated in 2018 to be a more user-friendly and easy-operable application.
Aims
To evaluate if disease modifying treatment (DMT) practice across centres participating in BSR is similar and what the mortality rate is among patients with sickle cell disease (SCD) included in the BSR.
Methods
All data from the initial 2008 database cohort were retrospectively encoded in the updated database format. All new data are recorded prospectively from neonatal screening or first contact until last annual follow-up (FU) or death. The data collected included diagnosis, demography, treatment and outcome data. Data were extracted from the database in January 2021 and were registered by 13 different centres.
Results
There were 977 patients registered with a median FU of 9 years (1-53 y). Median age at last FU was 13 (0-60 y). 654 (87,5%) have a severe phenotype (SS or Sβ°) and 51% are female. Among them, 546 (56%) were born in Belgium of which 369 (68%) were diagnosed by neonatal screening. In the absence of a neonatal screening, median age at diagnosis was 1 year (range 0-18). At last follow-up, 707 patients (72.3%) receive DMT: 523 patients receive hydroxyurea (HU), 126 underwent hematopoietic stem cell transplantation (HSCT), 53 are chronically transfused, 5 participate to a study with crizanlizumab.
Among not transplanted patients, the proportion of those receiving HU is significantly different among centres (46% to 75% ; p<0,0001). The median age at which HU was initiated was also significantly different among the centres (4y to 21y; p<0,0001).
Most of HSCT were performed in two main centres i.e., 66 and 58, respectively. Median age at HSCT was significantly different between both centres ( 8y (2-15) versus 5y (0-19) ; p=0,004).
Twenty-four (2.5%) patients died which account for a mortality rate of 0.22/100 patients-years (PY) and a trend to increase with age (0,18/100PY < 18 years, 0.39/100PY 18 -40 years and 0.63/100PY > 40 years ; p=0.056) .
Kaplan-Meier estimate of survival at 20 years is similar regardless of the mode of diagnosis (89%, 95%, 92% respectively for neonatal screening, not screened and not born in Belgium; p=0.260)(Figure 1).

Conclusion
Among the patient cohort recorded in the BSR, the proportion of patients receiving DMT, the age at start of HU and the age at HSCT is significantly different between centres. A detailed analysis of practices based at least on demographic criteria, severity of the disease, etc. is needed to understand their causes. Mortality remains low but with a trend to increase with age. The absence of a national neonatal screening program, deaths occurring before diagnosis and no inclusion in the registry, represent a bias in the interpretation of the mortality. Survival status might be impacted by the proportion of patients that consented to participate to the BSR and could be different between the neonatal screening, born in Belgium not screened, and born abroad groups.
Keyword(s): HSCT, Hydroxyurea, Mortality, Sickle cell disease


