
Contributions
Abstract: EP1206
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is an inherited disorder in which sickle hemoglobin (HbS) polymerization triggers red blood cell sickling, chronic hemolysis, anemia, and episodic vaso-occlusion. In addition to acute life-threatening events, SCD-related complications also include cumulative organ damage and early mortality. Voxelotor is an oral HbS polymerization inhibitor that increases hemoglobin (Hb) levels and reduces markers of hemolysis, and is approved in the United States for treatment of SCD in patients ≥12 years of age.
Aims
To characterize the real-world hematologic and clinical responses to voxelotor treatment in a multicenter, retrospective series of patients with SCD.
Methods
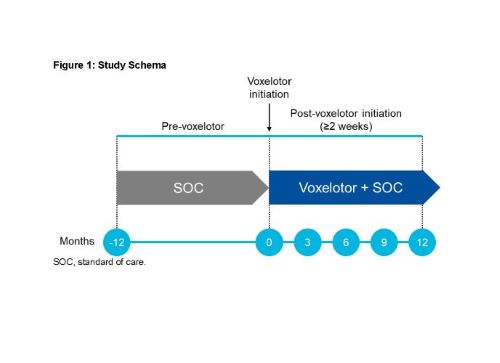
We collected and aggregated real-world, retrospective laboratory and clinical data on adolescents and adults (aged ≥12 years) with SCD treated with voxelotor as part of their usual care at multiple clinical centers in the United States. Laboratory and clinical data were collected 12 months before initiation of voxelotor and compared to similarly collected post-treatment data outcomes (Figure 1). Included patients received voxelotor for ≥2 consecutive weeks. Only data that were available from the patients’ medical records and other secondary data sources were collected. Study data from 1 year before and up to 1 year after the first dose of voxelotor were entered in de-identified case report forms via an electronic data capture system.
Results
Approximately 300 patients have been treated across the 9 clinical centers participating in this study. Changes from the pre-voxelotor treatment period in Hb, hemolysis markers, and iron overload will be summarized descriptively. Changes in the annualized incidence rates of significant SCD-related clinical events from the pre-voxelotor treatment period will be reported, including vaso-occlusive crises, acute chest syndrome, priapism, cerebral infarcts (overt and silent), transient ischemic attack, leg ulcers, and measures of cardiac function, renal function, and tricuspid regurgitation velocity/pulmonary hypertension. The associations between changes in Hb, markers of hemolysis, and incidences of SCD-related clinical events will be evaluated. Health resource utilization changes from the pre-voxelotor treatment period will be reported, including acute clinic visits, emergency department visits, hospitalizations, acute and chronic transfusions, and home oxygen supplementation. Changes in health-related quality-of-life measures collected as part of usual care will be summarized descriptively. Adverse events by severity and relationship to voxelotor will be collected and tabulated.
Conclusion
This study is the first to examine the real-world effectiveness of voxelotor in a multicenter, retrospective study and describe the observed changes in laboratory and clinical outcomes after at least 2 weeks of therapy in up to 300 patients.
Keyword(s): Hemolysis, Outcome, Patient, Sickle cell disease
Abstract: EP1206
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is an inherited disorder in which sickle hemoglobin (HbS) polymerization triggers red blood cell sickling, chronic hemolysis, anemia, and episodic vaso-occlusion. In addition to acute life-threatening events, SCD-related complications also include cumulative organ damage and early mortality. Voxelotor is an oral HbS polymerization inhibitor that increases hemoglobin (Hb) levels and reduces markers of hemolysis, and is approved in the United States for treatment of SCD in patients ≥12 years of age.
Aims
To characterize the real-world hematologic and clinical responses to voxelotor treatment in a multicenter, retrospective series of patients with SCD.
Methods
We collected and aggregated real-world, retrospective laboratory and clinical data on adolescents and adults (aged ≥12 years) with SCD treated with voxelotor as part of their usual care at multiple clinical centers in the United States. Laboratory and clinical data were collected 12 months before initiation of voxelotor and compared to similarly collected post-treatment data outcomes (Figure 1). Included patients received voxelotor for ≥2 consecutive weeks. Only data that were available from the patients’ medical records and other secondary data sources were collected. Study data from 1 year before and up to 1 year after the first dose of voxelotor were entered in de-identified case report forms via an electronic data capture system.
Results
Approximately 300 patients have been treated across the 9 clinical centers participating in this study. Changes from the pre-voxelotor treatment period in Hb, hemolysis markers, and iron overload will be summarized descriptively. Changes in the annualized incidence rates of significant SCD-related clinical events from the pre-voxelotor treatment period will be reported, including vaso-occlusive crises, acute chest syndrome, priapism, cerebral infarcts (overt and silent), transient ischemic attack, leg ulcers, and measures of cardiac function, renal function, and tricuspid regurgitation velocity/pulmonary hypertension. The associations between changes in Hb, markers of hemolysis, and incidences of SCD-related clinical events will be evaluated. Health resource utilization changes from the pre-voxelotor treatment period will be reported, including acute clinic visits, emergency department visits, hospitalizations, acute and chronic transfusions, and home oxygen supplementation. Changes in health-related quality-of-life measures collected as part of usual care will be summarized descriptively. Adverse events by severity and relationship to voxelotor will be collected and tabulated.

Conclusion
This study is the first to examine the real-world effectiveness of voxelotor in a multicenter, retrospective study and describe the observed changes in laboratory and clinical outcomes after at least 2 weeks of therapy in up to 300 patients.
Keyword(s): Hemolysis, Outcome, Patient, Sickle cell disease


