
Contributions
Abstract: EP1205
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is highly prevalent across sub-Saharan Africa and is a significant source of under-5 mortality. However, accurate characterization of the prevalence is difficult to determine due to the absence of systematic newborn screening in most African countries. Mali is a land-locked West African country with a high burden of sickle cell disease. Despite the critical need for early diagnosis of SCD to improve survival, wide scale diagnostic efforts utilizing isoelectric focusing (IEF) or high-performance liquid chromatography (HPLC) in sub-Saharan Africa have been limited by a paucity of laboratory equipment and trained personnel, unreliable supply chain for consumables, and overall expense. Point-of-Care (POC) testing for SCD is advancing rapidly and has the potential to reduce these barriers.
Aims
The objective of this study was to assess the feasibility and efficacy of systematic, POC newborn screening for SCD at a rural hospital in Mali, West Africa.
Methods
Between March 2019 and Jan 2021, all newborns at Koutiala Women’s and Children’s Hospital were offered screening for SCD with the Hemotype SC™ POC test kit (Silver Lake Research, Azusa, California, USA). Each kit included a plastic test tube, blood sampling device, and Elisa-based test strip. Verbal consent was obtained and heel-stick samples were procured within the first day of life. The samples were processed according to the package insert. The testing required 6 drops of unfiltered tap water. No other reagents or equipment were necessary. The results were available within 10 minutes and were provided to the caregiver prior to discharge. Newborns who were found to have sickle cell disease were referred to the SCD outpatient treatment program for ongoing management. Families of those with HbS trait or HbC trait received appropriate genetic counseling.
Results
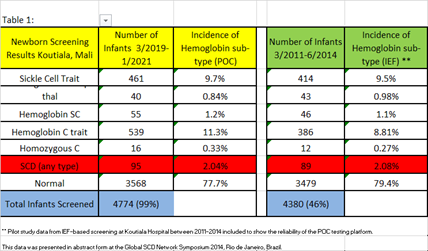
4822 infants were born over the study period and 4774 (99%) received POC screening. Screening results revealed Hemoglobin S trait; n=461(9.7%), Hemoglobin C trait; n=539 (11.3%), Hemoglobin SS; n=40 (0.84%), Hemoglobin SC; n=55 (1.2%), and homozygous C; n=16 (0.33%). In all, 95 infants (2.04%) were found to have sickle cell disease and were referred to the SCD outpatient treatment program. In addition, 22% of infants were found to be carriers of either HbC trait or HbS trait. The POC testing results were directly compared to historical pilot data of IEF-based SCD newborn screening at Koutiala Hospital between 2011-2014 (Table 1).
Conclusion
This pilot study is the first to examine the impact of systematic POC infant screening for SCD in Mali, West Africa. The rapidity of the POC testing resulted in a high newborn screening rate and increased the percentage of SCD-affected infants who successfully transitioned into the outpatient treatment program at the hospital. The POC testing results were very comparable and highly feasible when reviewed alongside historical IEF-based screening at Koutiala Hospital. While the incidence of hemoglobin abnormalities discovered by newborn screening was very similar between these different testing strategies, the percentage of evaluable births that were screened was much higher in this POC cohort than in the IEF pilot study (99% vs 46%). Overall, the low cost (<$2/test), ease of use, and reliability of the Hemotype SC™ POC test has the potential to make it a more desirable option for wider scale screening efforts in the future.
Keyword(s): Infant, Point-of-care, Screening, Sickle cell
Abstract: EP1205
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is highly prevalent across sub-Saharan Africa and is a significant source of under-5 mortality. However, accurate characterization of the prevalence is difficult to determine due to the absence of systematic newborn screening in most African countries. Mali is a land-locked West African country with a high burden of sickle cell disease. Despite the critical need for early diagnosis of SCD to improve survival, wide scale diagnostic efforts utilizing isoelectric focusing (IEF) or high-performance liquid chromatography (HPLC) in sub-Saharan Africa have been limited by a paucity of laboratory equipment and trained personnel, unreliable supply chain for consumables, and overall expense. Point-of-Care (POC) testing for SCD is advancing rapidly and has the potential to reduce these barriers.
Aims
The objective of this study was to assess the feasibility and efficacy of systematic, POC newborn screening for SCD at a rural hospital in Mali, West Africa.
Methods
Between March 2019 and Jan 2021, all newborns at Koutiala Women’s and Children’s Hospital were offered screening for SCD with the Hemotype SC™ POC test kit (Silver Lake Research, Azusa, California, USA). Each kit included a plastic test tube, blood sampling device, and Elisa-based test strip. Verbal consent was obtained and heel-stick samples were procured within the first day of life. The samples were processed according to the package insert. The testing required 6 drops of unfiltered tap water. No other reagents or equipment were necessary. The results were available within 10 minutes and were provided to the caregiver prior to discharge. Newborns who were found to have sickle cell disease were referred to the SCD outpatient treatment program for ongoing management. Families of those with HbS trait or HbC trait received appropriate genetic counseling.
Results
4822 infants were born over the study period and 4774 (99%) received POC screening. Screening results revealed Hemoglobin S trait; n=461(9.7%), Hemoglobin C trait; n=539 (11.3%), Hemoglobin SS; n=40 (0.84%), Hemoglobin SC; n=55 (1.2%), and homozygous C; n=16 (0.33%). In all, 95 infants (2.04%) were found to have sickle cell disease and were referred to the SCD outpatient treatment program. In addition, 22% of infants were found to be carriers of either HbC trait or HbS trait. The POC testing results were directly compared to historical pilot data of IEF-based SCD newborn screening at Koutiala Hospital between 2011-2014 (Table 1).

Conclusion
This pilot study is the first to examine the impact of systematic POC infant screening for SCD in Mali, West Africa. The rapidity of the POC testing resulted in a high newborn screening rate and increased the percentage of SCD-affected infants who successfully transitioned into the outpatient treatment program at the hospital. The POC testing results were very comparable and highly feasible when reviewed alongside historical IEF-based screening at Koutiala Hospital. While the incidence of hemoglobin abnormalities discovered by newborn screening was very similar between these different testing strategies, the percentage of evaluable births that were screened was much higher in this POC cohort than in the IEF pilot study (99% vs 46%). Overall, the low cost (<$2/test), ease of use, and reliability of the Hemotype SC™ POC test has the potential to make it a more desirable option for wider scale screening efforts in the future.
Keyword(s): Infant, Point-of-care, Screening, Sickle cell


