
Contributions
Abstract: EP1199
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Management of patients with sickle cell disease (SCD) in the UK relies largely on transfusion therapy and hydroxycarbamide alongside supportive care. New therapies to improve morbidity and mortality are needed. Two new therapies undergoing NICE technology appraisal are Crizanlizumab and Voxelotor. Crizanlizumab, a p-selectin inhibitor, has been shown to reduce the frequency of vaso-occlusive crisis in patients aged 16 or over in a randomised, placebo-controlled phase 2 trial (SUSTAIN)1. Voxelotor is an HbS polymerisation inhibitor and showed increased haemoglobin levels with reduced markers of haemolysis in a phase 3 trial (HOPE)2. Both were approved by the FDA in November 2019.
Aims
The eligibility criteria used in the SUSTAIN and HOPE trials have been compared against the Bristol Haematology and Oncology Centre and Oxford University Hospitals SCD cohorts to identify those patients who would be eligible for these emerging therapies.
Methods
The patient databases were cross-referenced with electronic notes to identify eligibility data. Vaso-occlusive crises (VOC) were determined by calls to the haemoglobinopathy team or helpline, attendance at the Acute Haematology Unit or admission. Electronic blood results were checked to ensure haemoglobin fell within the specified ranges.
Results
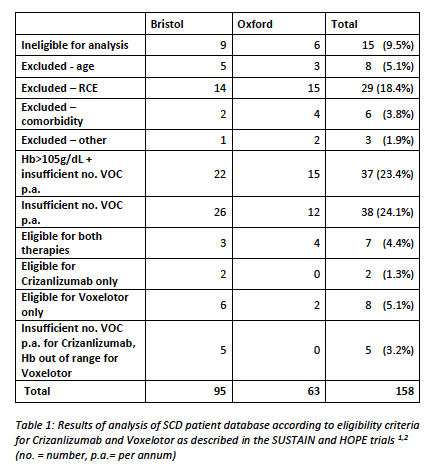
Results of analysis of SCD patient database according to eligibility criteria for Crizanlizumab and Voxelotor as described in the SUSTAIN and HOPE trials 1,2 are shown in Table 1. Results show that, out of 158 patients with eligible sickle cell disorders, only 7 (4.4%) were eligible for both therapies. 8 (5.1%) were eligible for Voxelotor only, and 2 (1.3%) were only eligible for Crizanlizumab. Patients with no VOC in 12 months were ineligible for either therapy (n=75, 47.5%) and just under half of these patients had a baseline haemoglobin over the range of eligibility for Voxelotor (n=37, 23.4%). 5 patients with a single VOC in 12 months had an Hb out of range for Voxelotor (3.2%). Regular transfusion therapy was the second most common exclusion (n=29, 18.3%). Age over 65 excluded 8 (5.1%) of our patients, and 6 (3.8%) had significant comorbidity that rendered them ineligible. 15 (9.5%) of our patients had moved out of area so estimates of VOC per year are likely to be inaccurate. Other reasons for exclusion included titration of hydroxycarbamide and family planning.
Conclusion
Though new therapies are being developed for prevention of VOC in SCD, few patients in our cohort are eligible for these. Incidence of severe VOC may be underestimated as patients self-manage at home, particularly in the current Covid-19 pandemic. This should be kept under review and closer liaison with patients about their VOC may help identify eligible patients. Strict adherence to eligibility based on that of clinical trials is likely to result in very few patients benefitting from these new therapies.
Keyword(s): P-selectin, Sickle cell disease, Treatment, Vasoocclusive crisis
Abstract: EP1199
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Management of patients with sickle cell disease (SCD) in the UK relies largely on transfusion therapy and hydroxycarbamide alongside supportive care. New therapies to improve morbidity and mortality are needed. Two new therapies undergoing NICE technology appraisal are Crizanlizumab and Voxelotor. Crizanlizumab, a p-selectin inhibitor, has been shown to reduce the frequency of vaso-occlusive crisis in patients aged 16 or over in a randomised, placebo-controlled phase 2 trial (SUSTAIN)1. Voxelotor is an HbS polymerisation inhibitor and showed increased haemoglobin levels with reduced markers of haemolysis in a phase 3 trial (HOPE)2. Both were approved by the FDA in November 2019.
Aims
The eligibility criteria used in the SUSTAIN and HOPE trials have been compared against the Bristol Haematology and Oncology Centre and Oxford University Hospitals SCD cohorts to identify those patients who would be eligible for these emerging therapies.
Methods
The patient databases were cross-referenced with electronic notes to identify eligibility data. Vaso-occlusive crises (VOC) were determined by calls to the haemoglobinopathy team or helpline, attendance at the Acute Haematology Unit or admission. Electronic blood results were checked to ensure haemoglobin fell within the specified ranges.
Results
Results of analysis of SCD patient database according to eligibility criteria for Crizanlizumab and Voxelotor as described in the SUSTAIN and HOPE trials 1,2 are shown in Table 1. Results show that, out of 158 patients with eligible sickle cell disorders, only 7 (4.4%) were eligible for both therapies. 8 (5.1%) were eligible for Voxelotor only, and 2 (1.3%) were only eligible for Crizanlizumab. Patients with no VOC in 12 months were ineligible for either therapy (n=75, 47.5%) and just under half of these patients had a baseline haemoglobin over the range of eligibility for Voxelotor (n=37, 23.4%). 5 patients with a single VOC in 12 months had an Hb out of range for Voxelotor (3.2%). Regular transfusion therapy was the second most common exclusion (n=29, 18.3%). Age over 65 excluded 8 (5.1%) of our patients, and 6 (3.8%) had significant comorbidity that rendered them ineligible. 15 (9.5%) of our patients had moved out of area so estimates of VOC per year are likely to be inaccurate. Other reasons for exclusion included titration of hydroxycarbamide and family planning.

Conclusion
Though new therapies are being developed for prevention of VOC in SCD, few patients in our cohort are eligible for these. Incidence of severe VOC may be underestimated as patients self-manage at home, particularly in the current Covid-19 pandemic. This should be kept under review and closer liaison with patients about their VOC may help identify eligible patients. Strict adherence to eligibility based on that of clinical trials is likely to result in very few patients benefitting from these new therapies.
Keyword(s): P-selectin, Sickle cell disease, Treatment, Vasoocclusive crisis


