
Contributions
Abstract: EP1198
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is a genetic disorder caused by the inheritance of two alleles bearing a single nucleotide change in the β globin gene coding sequence. The pathophysiologic mechanism of SCD involves polymerization of intracellular sickle hemoglobin (HbS) following deoxygenation in the microvasculature, leading to decreased red blood cell (RBC) deformability, morphologic sickling of RBCs, decreased RBC survival, microvascular obstruction, and clinical complications. Voxelotor, a HbS polymerization inhibitor recently approved for the treatment of SCD in the United States, is an allosteric modifier of hemoglobin (Hb) that increases the proportion of oxygenated Hb in all RBCs. In clinical studies in patients with SCD, voxelotor demonstrated that doses of up to 1500 mg daily achieved Hb modifications of ~27%, was well tolerated and resulted in reduced hemolytic anemia. GBT021601 is a potent second generation HbS polymerization inhibitor with the potential to achieve even higher Hb modification in patients with SCD at lower doses and therefore with less pill burden.
Aims
To evaluate the effect of GBT021601, a second generation HbS polymerization inhibitor, in mouse model of SCD.
Methods
To evaluate its effect on the pathophysiology of SCD, GBT021601 was orally administered at 20, 40, 75, and 150 mg/kg once-daily doses for 21 days in Townes SCD mice (SS mice).
Results
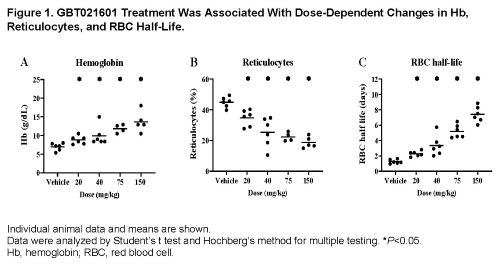
At steady state, GBT021601-treated SS mice achieved Hb occupancies (at minimum concentration in plasma) of 6%, 12%, 21%, and 29% corresponding to 20, 40, 75, and 150 mg/kg doses, respectively. This resulted in a dose-dependent reduction in p50, partial pressure of oxygen at 50% Hb saturation, of SS mouse blood from ~28 mmHg (vehicle-treated) to 11 mmHg at the highest dose tested. Consequently, GBT021601 significantly reduced in vivo circulating sickled cells and ex vivo sickling of SS mouse blood under hypoxic conditions (pO2, 20 mmHg). Consistent with its anti-polymerization activity, GBT021601 reduced hemolysis as demonstrated by: 1.) an increase in Hb of up to 6.7 g/dL (Figure 1A), thereby achieving the normal range for wildtype mice, and 2.) a reduction in the percentage of reticulocytes of up to 58% relative to vehicle-treated SS mice (Figure 1B). Additionally, GBT021601 treatment increased the percentage of mature RBCs, reduced the percentage of mitochondria-containing RBCs, and improved RBC deformability, collectively indicating an overall improvement in RBC health. Consistent with improved Hb levels, reduced hemolysis, and improved RBC health, GBT021601 dose-dependently increased RBC half-life from 1.3 days in vehicle-treated SS mice to 7.4 days in SS mice treated with 150 mg/kg GBT021601 (Figure 1C). Furthermore, treatment of SS mice with GBT021601 for 21 days led to a significant reduction in spleen weight, indicating an improvement in splenic blood flow and organ function. Lastly, GBT021601 treatment for longer than 21 days (up to 42 days) indicated a dose-duration dependent improvement in hemolysis markers including Hb level and reticulocyte count.
Conclusion
Together, these data demonstrate that GBT021601, a next generation HbS polymerization inhibitor, normalized Hb, reduced hemolysis, and significantly improved RBC health, RBC half-life, and organ function in a mouse model of SCD. These data support clinical development of GBT021601 as a potential best-in-class HbS polymerization inhibitor for patients with SCD.
Keyword(s): Hemoglobin, Hemolytic anemia, Mouse model, Sickle cell disease
Abstract: EP1198
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is a genetic disorder caused by the inheritance of two alleles bearing a single nucleotide change in the β globin gene coding sequence. The pathophysiologic mechanism of SCD involves polymerization of intracellular sickle hemoglobin (HbS) following deoxygenation in the microvasculature, leading to decreased red blood cell (RBC) deformability, morphologic sickling of RBCs, decreased RBC survival, microvascular obstruction, and clinical complications. Voxelotor, a HbS polymerization inhibitor recently approved for the treatment of SCD in the United States, is an allosteric modifier of hemoglobin (Hb) that increases the proportion of oxygenated Hb in all RBCs. In clinical studies in patients with SCD, voxelotor demonstrated that doses of up to 1500 mg daily achieved Hb modifications of ~27%, was well tolerated and resulted in reduced hemolytic anemia. GBT021601 is a potent second generation HbS polymerization inhibitor with the potential to achieve even higher Hb modification in patients with SCD at lower doses and therefore with less pill burden.
Aims
To evaluate the effect of GBT021601, a second generation HbS polymerization inhibitor, in mouse model of SCD.
Methods
To evaluate its effect on the pathophysiology of SCD, GBT021601 was orally administered at 20, 40, 75, and 150 mg/kg once-daily doses for 21 days in Townes SCD mice (SS mice).
Results
At steady state, GBT021601-treated SS mice achieved Hb occupancies (at minimum concentration in plasma) of 6%, 12%, 21%, and 29% corresponding to 20, 40, 75, and 150 mg/kg doses, respectively. This resulted in a dose-dependent reduction in p50, partial pressure of oxygen at 50% Hb saturation, of SS mouse blood from ~28 mmHg (vehicle-treated) to 11 mmHg at the highest dose tested. Consequently, GBT021601 significantly reduced in vivo circulating sickled cells and ex vivo sickling of SS mouse blood under hypoxic conditions (pO2, 20 mmHg). Consistent with its anti-polymerization activity, GBT021601 reduced hemolysis as demonstrated by: 1.) an increase in Hb of up to 6.7 g/dL (Figure 1A), thereby achieving the normal range for wildtype mice, and 2.) a reduction in the percentage of reticulocytes of up to 58% relative to vehicle-treated SS mice (Figure 1B). Additionally, GBT021601 treatment increased the percentage of mature RBCs, reduced the percentage of mitochondria-containing RBCs, and improved RBC deformability, collectively indicating an overall improvement in RBC health. Consistent with improved Hb levels, reduced hemolysis, and improved RBC health, GBT021601 dose-dependently increased RBC half-life from 1.3 days in vehicle-treated SS mice to 7.4 days in SS mice treated with 150 mg/kg GBT021601 (Figure 1C). Furthermore, treatment of SS mice with GBT021601 for 21 days led to a significant reduction in spleen weight, indicating an improvement in splenic blood flow and organ function. Lastly, GBT021601 treatment for longer than 21 days (up to 42 days) indicated a dose-duration dependent improvement in hemolysis markers including Hb level and reticulocyte count.

Conclusion
Together, these data demonstrate that GBT021601, a next generation HbS polymerization inhibitor, normalized Hb, reduced hemolysis, and significantly improved RBC health, RBC half-life, and organ function in a mouse model of SCD. These data support clinical development of GBT021601 as a potential best-in-class HbS polymerization inhibitor for patients with SCD.
Keyword(s): Hemoglobin, Hemolytic anemia, Mouse model, Sickle cell disease


