
Contributions
Abstract: EP1197
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is a severe, inherited hemoglobinopathy, resulting in poor quality of life and reduced life expectancy. Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only established curative treatment option for SCD. In adults, myeloablative conditioning is associated with significant toxicity. Matched sibling donor (MSD) transplantation with non-myeloablative conditioning (alemtuzumab/3Gy total body irradiation (TBI)) has shown promising results in adult SCD patients. Patients treated with this regimen had their sickle cell phenotype corrected with only mild complications and no reports of graft-versus-host disease (GvHD). However, a large part of these patients reached very low donor T-cell chimerism, resulting in 13% graft failure and necessitating chronic use of immunosuppression to avoid graft failure. We hypothesized that adding azathioprine (suppressing patient T-cells) and hydroxyurea (reducing bone marrow expansion) as preconditioning to the alemtuzumab/TBI regimen might improve donor chimerism and reduce risk of graft failure.
Aims
In this study we prospectively investigate the effects of azathioprine/hydroxyurea preconditioning on donor chimerism and graft failure in patients receiving non-myeloablative MSD HSCT for SCD.
Methods
Adult SCD patients with an MSD were eligible for this treatment. After 3 months of azathioprine 150mg qd and hydroxyurea 25mg/kg qd, erythrocyte exchange transfusion was performed on day –10. Alemtuzumab/TBI conditioning was started on day –7, as described by Hsieh et al (NEJM, 2009). Graft-vs-host-disease (GvHD) prophylaxis consisted of sirolimus.
Results
As of august 2020, eleven SCD patients (median age 26 (range 19-49) years) were transplanted. All patients engrafted successfully. After a median follow-up of 22 months, median donor myeloid and T-cell chimerism were 100% (range 84%>100%) and 68% (range 51%>88%), respectively. These donor chimerism percentages are higher than previously reported with alemtuzumab/TBI only. All patients had a corrected SCD phenotype with normalized hemoglobin levels. Patients reaching one year post-transplantation were able to stop sirolimus without decreases in chimerism. Only one patient developed acute grade II intestinal GvHD, that responded well to steroids. There were no viral reactivations or signs of macrophage activation syndrome.
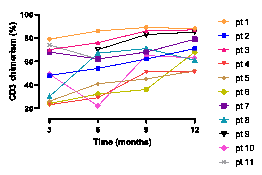
Conclusion
Azathioprine/hydroxyurea preconditioning prior to alemtuzumab/TBI results in improved donor chimerism, potentially reducing risk of graft failure after non-myeloablatieve MSD transplantation in SCD patients. Importantly, in contrast to earlier studies, all patients in this study were able to stop sirolimus as scheduled. Adding azathioprine/hydroxyurea preconditioning renders the alemtuzumab/TBI non-myeloablative HSCT, with low risk of transplantation-related toxicity, a viable alternative for adult SCD patients.
Keyword(s): Chimerism, Non-myeloablative, Sickle cell disease, Stem cell transplant
Abstract: EP1197
Type: E-Poster Presentation
Session title: Sickle cell disease
Background
Sickle cell disease (SCD) is a severe, inherited hemoglobinopathy, resulting in poor quality of life and reduced life expectancy. Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only established curative treatment option for SCD. In adults, myeloablative conditioning is associated with significant toxicity. Matched sibling donor (MSD) transplantation with non-myeloablative conditioning (alemtuzumab/3Gy total body irradiation (TBI)) has shown promising results in adult SCD patients. Patients treated with this regimen had their sickle cell phenotype corrected with only mild complications and no reports of graft-versus-host disease (GvHD). However, a large part of these patients reached very low donor T-cell chimerism, resulting in 13% graft failure and necessitating chronic use of immunosuppression to avoid graft failure. We hypothesized that adding azathioprine (suppressing patient T-cells) and hydroxyurea (reducing bone marrow expansion) as preconditioning to the alemtuzumab/TBI regimen might improve donor chimerism and reduce risk of graft failure.
Aims
In this study we prospectively investigate the effects of azathioprine/hydroxyurea preconditioning on donor chimerism and graft failure in patients receiving non-myeloablative MSD HSCT for SCD.
Methods
Adult SCD patients with an MSD were eligible for this treatment. After 3 months of azathioprine 150mg qd and hydroxyurea 25mg/kg qd, erythrocyte exchange transfusion was performed on day –10. Alemtuzumab/TBI conditioning was started on day –7, as described by Hsieh et al (NEJM, 2009). Graft-vs-host-disease (GvHD) prophylaxis consisted of sirolimus.
Results
As of august 2020, eleven SCD patients (median age 26 (range 19-49) years) were transplanted. All patients engrafted successfully. After a median follow-up of 22 months, median donor myeloid and T-cell chimerism were 100% (range 84%>100%) and 68% (range 51%>88%), respectively. These donor chimerism percentages are higher than previously reported with alemtuzumab/TBI only. All patients had a corrected SCD phenotype with normalized hemoglobin levels. Patients reaching one year post-transplantation were able to stop sirolimus without decreases in chimerism. Only one patient developed acute grade II intestinal GvHD, that responded well to steroids. There were no viral reactivations or signs of macrophage activation syndrome.

Conclusion
Azathioprine/hydroxyurea preconditioning prior to alemtuzumab/TBI results in improved donor chimerism, potentially reducing risk of graft failure after non-myeloablatieve MSD transplantation in SCD patients. Importantly, in contrast to earlier studies, all patients in this study were able to stop sirolimus as scheduled. Adding azathioprine/hydroxyurea preconditioning renders the alemtuzumab/TBI non-myeloablative HSCT, with low risk of transplantation-related toxicity, a viable alternative for adult SCD patients.
Keyword(s): Chimerism, Non-myeloablative, Sickle cell disease, Stem cell transplant


