
Contributions
Abstract: EP1196
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Chronic myeloid leukemia (CML) patient organisation representing countries of the CML Advocates Network Easter Europe and West Asia region have come to conclusion that they share similar issues in access to treatment and monitoring tools. The reason is the lack of a common database with the relevant information about CML.
Aims
The goal was to create the CML Atlas of Central and Eastern Europe and West Asia region to showcase the most relevant data on CML about access to tyrosine kinase inhibitors (TKIs) and monitoring tools to support CML patient organisations’ advocacy in those countries.
Methods
An European online survey was conducted, recruiting CML patient advocates and doctors from 19 countries, representing over 26.000 CML patients from Armenia, Croatia, Czech Republic, Georgia, Hungary, Kazakhstan, Kosovo, Kyrgyzstan, Lithuania, Moldova, North Macedonia, Poland, Russia, Serbia, Slovenia, Tajikistan, Turkey, Ukraine and Uzbekistan. The survey started on 1st December 2019 and lasted until 31st March 2020 covering the following topics: CML general information, availability of TKIs and PCR testing, clinical trials and treatment free remission data. Presented data as of 1st July 2020.
Results
19 countries reported Imatinib available, 13 of them on insurance. Collected data show that in Armenia, Georgia, Kyrgyzstan, Moldova, Tajikistan and Uzbekistan availability was managed by donations; in Kazakhstan, Imatinib was available both on insurance and donations and no countries reported its availability in clinical trials.
18 countries reported Nilotinib available, with the exception of Kosovo, although 12 countries declared it was just on insurance. Data from Armenia, Georgia, Kyrgyzstan, Moldova, Tajikistan and Uzbekistan show that Nilotinib was available on donations and no countries reported its use in clinical trials.
16 countries informed on Dasatinib availability: the drug was obtainable on insurance in 10 of them, and through donations in Armenia, Kyrgyzstan, Moldova, North Macedonia, Serbia and Tajikistan. No countries reported access to Dasatinib through clinical trials.
9 countries reported that Bosutinib was available on insurance. Therefore, in 10 countries of the region, this drug was not available for CML patients.
Ponatinib reported as an available drug on insurance in 6 countries as well as on donations, while in 2 countries was reported as available in clinical trials. This medicine was not available in 6 countries of the region.
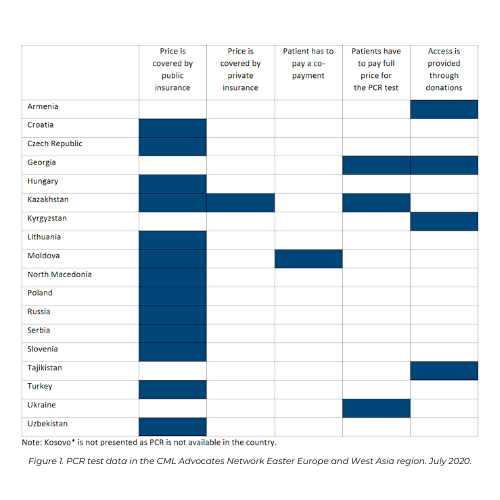
Collected data about testing demonstrate that PCR was available in all countries participating in the survey, except Kosovo. In the majority of countries, price of PCR is covered by public insurance. However, in countries as Armenia, Kyrgyzstan and Tajikistan access to PCR testing is provided through donations.
Conclusion
There are important differences in access to TKIs to treat CML patients between countries of the Central and Eastern Europe and West Asia region. While five TKIs are available on these countries, the majority of them are just available on insurance and/or donations. Access to free PCR testing for CML patients is not ensured in all countries. There are opportunities to improve access to high-quality and safe therapies and effective monitoring tools for CML patients in the region.
Keyword(s): Chronic myeloid leukemia, Patient, PCR, Tyrosine kinase inhibitor
Abstract: EP1196
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Chronic myeloid leukemia (CML) patient organisation representing countries of the CML Advocates Network Easter Europe and West Asia region have come to conclusion that they share similar issues in access to treatment and monitoring tools. The reason is the lack of a common database with the relevant information about CML.
Aims
The goal was to create the CML Atlas of Central and Eastern Europe and West Asia region to showcase the most relevant data on CML about access to tyrosine kinase inhibitors (TKIs) and monitoring tools to support CML patient organisations’ advocacy in those countries.
Methods
An European online survey was conducted, recruiting CML patient advocates and doctors from 19 countries, representing over 26.000 CML patients from Armenia, Croatia, Czech Republic, Georgia, Hungary, Kazakhstan, Kosovo, Kyrgyzstan, Lithuania, Moldova, North Macedonia, Poland, Russia, Serbia, Slovenia, Tajikistan, Turkey, Ukraine and Uzbekistan. The survey started on 1st December 2019 and lasted until 31st March 2020 covering the following topics: CML general information, availability of TKIs and PCR testing, clinical trials and treatment free remission data. Presented data as of 1st July 2020.
Results
19 countries reported Imatinib available, 13 of them on insurance. Collected data show that in Armenia, Georgia, Kyrgyzstan, Moldova, Tajikistan and Uzbekistan availability was managed by donations; in Kazakhstan, Imatinib was available both on insurance and donations and no countries reported its availability in clinical trials.
18 countries reported Nilotinib available, with the exception of Kosovo, although 12 countries declared it was just on insurance. Data from Armenia, Georgia, Kyrgyzstan, Moldova, Tajikistan and Uzbekistan show that Nilotinib was available on donations and no countries reported its use in clinical trials.
16 countries informed on Dasatinib availability: the drug was obtainable on insurance in 10 of them, and through donations in Armenia, Kyrgyzstan, Moldova, North Macedonia, Serbia and Tajikistan. No countries reported access to Dasatinib through clinical trials.
9 countries reported that Bosutinib was available on insurance. Therefore, in 10 countries of the region, this drug was not available for CML patients.
Ponatinib reported as an available drug on insurance in 6 countries as well as on donations, while in 2 countries was reported as available in clinical trials. This medicine was not available in 6 countries of the region.
Collected data about testing demonstrate that PCR was available in all countries participating in the survey, except Kosovo. In the majority of countries, price of PCR is covered by public insurance. However, in countries as Armenia, Kyrgyzstan and Tajikistan access to PCR testing is provided through donations.

Conclusion
There are important differences in access to TKIs to treat CML patients between countries of the Central and Eastern Europe and West Asia region. While five TKIs are available on these countries, the majority of them are just available on insurance and/or donations. Access to free PCR testing for CML patients is not ensured in all countries. There are opportunities to improve access to high-quality and safe therapies and effective monitoring tools for CML patients in the region.
Keyword(s): Chronic myeloid leukemia, Patient, PCR, Tyrosine kinase inhibitor


