
Contributions
Abstract: EP1194
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Pyruvate kinase (PK) deficiency is a rare, inherited disorder caused by autosomal recessive mutations in the PKLR gene, whereby a glycolytic defect causes reduced adenosine triphosphate levels and leads to hemolytic anemia. Patients with PK deficiency can experience serious complications associated with the disease and its treatment, including perinatal complications, iron overload, bone fractures, pulmonary hypertension, and liver cirrhosis. The current standard of care for PK deficiency is supportive, including blood transfusions, splenectomy, cholecystectomy, iron chelation therapy, and/or interventions for other disease-related morbidity. Recent studies have demonstrated that iron overload and need for chelation is common even in patients that do not require regular transfusions. There is no approved drug therapy for this condition. Although the clinical burden in patients with PK deficiency is well documented, the economic burden of long-term disease management, procedures, and clinical complications has not been investigated and is unknown.
Aims
This study aimed to estimate the lifetime burden of patients with PK deficiency, from the perspective of the healthcare payer, in the United States (US).
Methods
A micro-costing method was employed to determine the direct costs associated with the diagnosis, treatment and monitoring of PK deficiency, and complications over a patient’s lifetime. The occurrences and frequency of complications and monitoring were derived from scientific literature and verified with clinical experts. Patient mortality was based on a previously published report of the PK deficiency population. Cost inputs were derived from public databases (eg, Medicare physician fee schedule, Healthcare Cost and Utilization Project [HCUP]) and economic literature from a US payer perspective. Total costs were calculated as the sum of early pediatric (0–5 y), late pediatric (6–17 y), and adult phases. Adult patients were grouped based on transfusion status (RT, regularly transfused [≥6 transfusions per year]; NRT, not regularly transfused; NT, never transfused). A univariate sensitivity analysis, at 10% variance, was conducted to identify major cost drivers.
Results
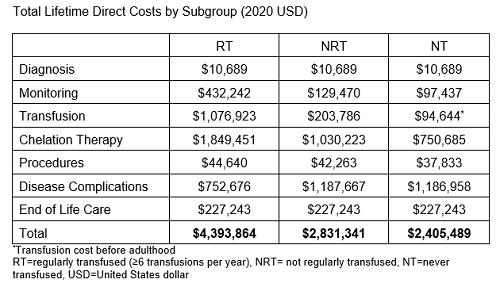
The mean lifetime direct cost of a patient with PK deficiency is $3.3 million with the majority of the costs occurring during the adult phase ($3.0 million). The mean lifetime cost varied by transfusion status with lifetime costs of $4.4 million, $2.8 million, and $2.4 million for RT, NRT, and NT patients, respectively (Table). Although transfusion and chelation costs were higher in those with a greater transfusion burden, the costs of complications, particularly from iron overload, were higher in the NRT and NT populations. The main cost-drivers overall were the adoption rate and cost of chelation therapies, the cost and frequency of transfusions, and the cost associated with complications.
Conclusion
The results of this study suggest that the lifetime economic burden of PK deficiency, from the perspective of the healthcare payer, is substantial. A large proportion of the direct cost of PK deficiency comes from transfusion and chelation therapies. Although the model provides a foundation for further economic evaluations, additional research regarding real-world healthcare resource utilization and indirect costs would further improve our understanding of the economic burden.
Keyword(s): Cost analysis, Health care, Hemolytic anemia, Pyruvate kinase deficiency
Abstract: EP1194
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Pyruvate kinase (PK) deficiency is a rare, inherited disorder caused by autosomal recessive mutations in the PKLR gene, whereby a glycolytic defect causes reduced adenosine triphosphate levels and leads to hemolytic anemia. Patients with PK deficiency can experience serious complications associated with the disease and its treatment, including perinatal complications, iron overload, bone fractures, pulmonary hypertension, and liver cirrhosis. The current standard of care for PK deficiency is supportive, including blood transfusions, splenectomy, cholecystectomy, iron chelation therapy, and/or interventions for other disease-related morbidity. Recent studies have demonstrated that iron overload and need for chelation is common even in patients that do not require regular transfusions. There is no approved drug therapy for this condition. Although the clinical burden in patients with PK deficiency is well documented, the economic burden of long-term disease management, procedures, and clinical complications has not been investigated and is unknown.
Aims
This study aimed to estimate the lifetime burden of patients with PK deficiency, from the perspective of the healthcare payer, in the United States (US).
Methods
A micro-costing method was employed to determine the direct costs associated with the diagnosis, treatment and monitoring of PK deficiency, and complications over a patient’s lifetime. The occurrences and frequency of complications and monitoring were derived from scientific literature and verified with clinical experts. Patient mortality was based on a previously published report of the PK deficiency population. Cost inputs were derived from public databases (eg, Medicare physician fee schedule, Healthcare Cost and Utilization Project [HCUP]) and economic literature from a US payer perspective. Total costs were calculated as the sum of early pediatric (0–5 y), late pediatric (6–17 y), and adult phases. Adult patients were grouped based on transfusion status (RT, regularly transfused [≥6 transfusions per year]; NRT, not regularly transfused; NT, never transfused). A univariate sensitivity analysis, at 10% variance, was conducted to identify major cost drivers.
Results
The mean lifetime direct cost of a patient with PK deficiency is $3.3 million with the majority of the costs occurring during the adult phase ($3.0 million). The mean lifetime cost varied by transfusion status with lifetime costs of $4.4 million, $2.8 million, and $2.4 million for RT, NRT, and NT patients, respectively (Table). Although transfusion and chelation costs were higher in those with a greater transfusion burden, the costs of complications, particularly from iron overload, were higher in the NRT and NT populations. The main cost-drivers overall were the adoption rate and cost of chelation therapies, the cost and frequency of transfusions, and the cost associated with complications.

Conclusion
The results of this study suggest that the lifetime economic burden of PK deficiency, from the perspective of the healthcare payer, is substantial. A large proportion of the direct cost of PK deficiency comes from transfusion and chelation therapies. Although the model provides a foundation for further economic evaluations, additional research regarding real-world healthcare resource utilization and indirect costs would further improve our understanding of the economic burden.
Keyword(s): Cost analysis, Health care, Hemolytic anemia, Pyruvate kinase deficiency


