
Contributions
Abstract: EP1182
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
At diagnosis, disease risk and transfusion burden (TB) can impact HRQoL in pts with MDS. The impact of disease status and higher transfusion requirements on HRQoL has not been well studied.
Aims
We used data from the Connect® Myeloid Disease Registry, an ongoing, prospective, observational cohort study that includes adult pts with lower-risk (LR) and higher-risk (HR) MDS, to investigate factors influencing baseline (BL) and subsequent HRQoL.
Methods
BL and Month 6 (M6) data from pts enrolled from Dec 12, 2013 to Mar 6, 2020 (data cutoff) were analyzed. Pts were stratified by International Prognostic Scoring System (IPSS) risk (LR, HR), treatment (Tx) within 45 days post-enrollment (no Tx, best supportive care [BSC], active Tx), and TB 16 weeks post-BL (non-transfusion dependent [NTD], low TB [LTB]; 1−3 transfusions, high TB [HTB]: ≥4 transfusions). Pts completed EuroQol 5 dimensions (EQ-5D), Functional Assessment of Cancer Therapy-Anemia (FACT-An) trial outcome index (TOI), and FACT-Fatigue (FACT-F) questionnaires at BL and quarterly thereafter. Clinically meaningful change, based on minimally important differences, was defined as a change of ±0.07 for EQ-5D, ±6 for FACT-An TOI, and ±3 for FACT-F.
Results
At data cutoff, 830 (489 LR, 341 HR) pts were enrolled. Median age was 74 years. 278 pts received no initial Tx, 161 BSC, and 378 active Tx. At BL, 470 were NTD, 197 LTB, and 163 HTB. Of 670 pts still on-study at M6, 462 completed the questionnaires at both BL and M6.
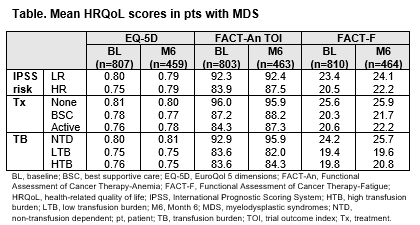
At BL, clinically meaningful differences were observed in FACT-An TOI and FACT-F scores, but not EQ-5D, between LR- and HR-MDS and the Tx subgroups. From BL to M6, no clinically meaningful changes were observed in mean scores for each questionnaire. For the TB subgroups, meaningful differences were observed at BL in FACT-An TOI and FACT-F scores, but not EQ-5D (Table). From BL to M6, meaningful decreases in scores were reported by 26%, 30%, and 35% of NTD, LTB, and HTB pts in EQ-5D, 41%, 43%, and 48% for FACT-An TOI, and 40%, 42%, and 48% for FACT-F; increases were reported by 19%, 19%, and 20% pts for EQ-5D, 31%, 32%, and 39% for FACT-An TOI, and 30%, 39%, and 40% for FACT-F.
Conclusion
This preliminary analysis suggests that pts with HR-MDS, and transfusion-dependent pts, generally had worse HRQoL at BL, providing further support to initiating active Tx in pts with TB. Possible limitations of the analysis are lower completion rates in pts with more severe disease, and EQ-5D may not capture changes in these subgroups at M6. A longer follow-up may help delineate the impact of Tx on HRQoL assessments in pts with MDS.
© 2021 American Society of Clinical Oncology, Inc. Reused with permission.
This abstract was accepted and previously presented at the 2021 ASCO Annual Meeting. All rights reserved.
Keyword(s): Myelodysplasia, Quality of life
Abstract: EP1182
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
At diagnosis, disease risk and transfusion burden (TB) can impact HRQoL in pts with MDS. The impact of disease status and higher transfusion requirements on HRQoL has not been well studied.
Aims
We used data from the Connect® Myeloid Disease Registry, an ongoing, prospective, observational cohort study that includes adult pts with lower-risk (LR) and higher-risk (HR) MDS, to investigate factors influencing baseline (BL) and subsequent HRQoL.
Methods
BL and Month 6 (M6) data from pts enrolled from Dec 12, 2013 to Mar 6, 2020 (data cutoff) were analyzed. Pts were stratified by International Prognostic Scoring System (IPSS) risk (LR, HR), treatment (Tx) within 45 days post-enrollment (no Tx, best supportive care [BSC], active Tx), and TB 16 weeks post-BL (non-transfusion dependent [NTD], low TB [LTB]; 1−3 transfusions, high TB [HTB]: ≥4 transfusions). Pts completed EuroQol 5 dimensions (EQ-5D), Functional Assessment of Cancer Therapy-Anemia (FACT-An) trial outcome index (TOI), and FACT-Fatigue (FACT-F) questionnaires at BL and quarterly thereafter. Clinically meaningful change, based on minimally important differences, was defined as a change of ±0.07 for EQ-5D, ±6 for FACT-An TOI, and ±3 for FACT-F.
Results
At data cutoff, 830 (489 LR, 341 HR) pts were enrolled. Median age was 74 years. 278 pts received no initial Tx, 161 BSC, and 378 active Tx. At BL, 470 were NTD, 197 LTB, and 163 HTB. Of 670 pts still on-study at M6, 462 completed the questionnaires at both BL and M6.
At BL, clinically meaningful differences were observed in FACT-An TOI and FACT-F scores, but not EQ-5D, between LR- and HR-MDS and the Tx subgroups. From BL to M6, no clinically meaningful changes were observed in mean scores for each questionnaire. For the TB subgroups, meaningful differences were observed at BL in FACT-An TOI and FACT-F scores, but not EQ-5D (Table). From BL to M6, meaningful decreases in scores were reported by 26%, 30%, and 35% of NTD, LTB, and HTB pts in EQ-5D, 41%, 43%, and 48% for FACT-An TOI, and 40%, 42%, and 48% for FACT-F; increases were reported by 19%, 19%, and 20% pts for EQ-5D, 31%, 32%, and 39% for FACT-An TOI, and 30%, 39%, and 40% for FACT-F.

Conclusion
This preliminary analysis suggests that pts with HR-MDS, and transfusion-dependent pts, generally had worse HRQoL at BL, providing further support to initiating active Tx in pts with TB. Possible limitations of the analysis are lower completion rates in pts with more severe disease, and EQ-5D may not capture changes in these subgroups at M6. A longer follow-up may help delineate the impact of Tx on HRQoL assessments in pts with MDS.
© 2021 American Society of Clinical Oncology, Inc. Reused with permission.
This abstract was accepted and previously presented at the 2021 ASCO Annual Meeting. All rights reserved.
Keyword(s): Myelodysplasia, Quality of life


