
Contributions
Abstract: EP1179
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Sutimlimab (formerly BIVV009) is a humanized monoclonal anti-C1s antibody with a clinical trial program to support its development as a treatment for cold agglutinin disease (CAD). CARDINAL (NCT03347396) and CADENZA (NCT03347422) are Phase 3 clinical trials for sutimlimab in patients with CAD. As fatigue is one of the most common symptoms of CAD (Su et al. ASH 2020; 2484), one of the secondary aims of CARDINAL and CADENZA was to examine treatment-related change in fatigue using the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue outcome measure.
Aims
To estimate the clinically important change (CIC) for FACIT-Fatigue in patients with CAD using pooled data from CARDINAL and CADENZA. The CIC reflects the smallest score change that indicates a meaningful treatment benefit for individual patients.
Methods
CARDINAL is an open-label, single-arm, multicenter study in patients with CAD with a recent blood transfusion. CADENZA is a randomized, double-blinded, placebo-controlled study in patients with CAD without a recent blood transfusion. Data from the Part A, 26-week component of both studies were combined for these analyses. Anchor- and distribution-based analyses were performed to estimate the CIC. Anchor-based approaches (mean change and model-based) examined the relationship between change in FACIT-Fatigue scores and change in related anchor variables from baseline to Week 26. The independent variable was change in FACIT-Fatigue score and dependent variables were binary (improvement vs. no improvement). The anchor variables were change in self-assessed general health perceptions (as measured through change in response to the general health scale [GH01] of the Short Form 12-Item (version 2)® Health Survey [SF-12v2]), change in Patient Global Impression of (fatigue) Severity (PGI-S), Patient Global Impression of Change (PGIC), and change in hemoglobin levels.
Results
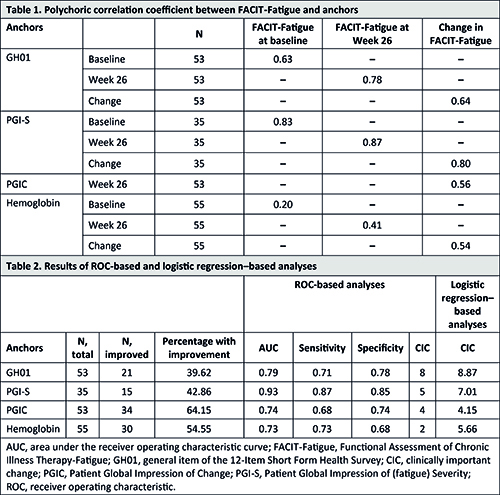
Fifty-five patients were included from CARDINAL (n=17) and CADENZA (n=38 [n=19 placebo, n=19 sutimlimab]). The median (range) age was 70 (46–88) years and 76% (n=42) were female. Mean FACIT-Fatigue score rose from 32.6 at baseline to 39.3 at Week 26, an improvement of 6.7 points. Correlations between FACIT-Fatigue scores and GH01, PGI-S, and PGIC exceeded 0.40, indicating moderately strong associations (Table 1). When using an anchor-based approach that evaluates mean change in FACIT-Fatigue score, CIC estimates ranged from 9.20 (PGIC) to 15.69 (PGI-S). Results from the two model-based anchor approaches that estimate CICs using receiver operating characteristic–based and logistic regression–based analyses ranged from 2 (hemoglobin) to 8.87 (GH01) (Table 2). CIC estimates generated from the anchor-based approaches ranged from 2 to 16 (interquartile range [IQR], 5–10). Distribution-based analyses determined the CIC to be 5.62 when based on one-half standard deviation of FACIT-Fatigue at baseline, and 2.78 when based on the standard error of the measure. The empirically determined CIC for patients with CAD for FACIT-Fatigue was 5, a median estimate produced by model-based analyses that falls within the IQR of all anchor-based analyses and converges with estimates produced by distribution-based analyses.
Conclusion
This study estimated a CIC of 5 for FACIT-Fatigue in patients with CAD, a CIC consistent with estimates for other disease areas (eg, rheumatoid arthritis, systemic lupus erythematosus, anemia related to cancer). This analysis demonstrated that the FACIT-Fatigue Scale may be an appropriate and interpretable assessment of fatigue among patients with CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Outcome, Quality of life
Abstract: EP1179
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
Sutimlimab (formerly BIVV009) is a humanized monoclonal anti-C1s antibody with a clinical trial program to support its development as a treatment for cold agglutinin disease (CAD). CARDINAL (NCT03347396) and CADENZA (NCT03347422) are Phase 3 clinical trials for sutimlimab in patients with CAD. As fatigue is one of the most common symptoms of CAD (Su et al. ASH 2020; 2484), one of the secondary aims of CARDINAL and CADENZA was to examine treatment-related change in fatigue using the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue outcome measure.
Aims
To estimate the clinically important change (CIC) for FACIT-Fatigue in patients with CAD using pooled data from CARDINAL and CADENZA. The CIC reflects the smallest score change that indicates a meaningful treatment benefit for individual patients.
Methods
CARDINAL is an open-label, single-arm, multicenter study in patients with CAD with a recent blood transfusion. CADENZA is a randomized, double-blinded, placebo-controlled study in patients with CAD without a recent blood transfusion. Data from the Part A, 26-week component of both studies were combined for these analyses. Anchor- and distribution-based analyses were performed to estimate the CIC. Anchor-based approaches (mean change and model-based) examined the relationship between change in FACIT-Fatigue scores and change in related anchor variables from baseline to Week 26. The independent variable was change in FACIT-Fatigue score and dependent variables were binary (improvement vs. no improvement). The anchor variables were change in self-assessed general health perceptions (as measured through change in response to the general health scale [GH01] of the Short Form 12-Item (version 2)® Health Survey [SF-12v2]), change in Patient Global Impression of (fatigue) Severity (PGI-S), Patient Global Impression of Change (PGIC), and change in hemoglobin levels.
Results
Fifty-five patients were included from CARDINAL (n=17) and CADENZA (n=38 [n=19 placebo, n=19 sutimlimab]). The median (range) age was 70 (46–88) years and 76% (n=42) were female. Mean FACIT-Fatigue score rose from 32.6 at baseline to 39.3 at Week 26, an improvement of 6.7 points. Correlations between FACIT-Fatigue scores and GH01, PGI-S, and PGIC exceeded 0.40, indicating moderately strong associations (Table 1). When using an anchor-based approach that evaluates mean change in FACIT-Fatigue score, CIC estimates ranged from 9.20 (PGIC) to 15.69 (PGI-S). Results from the two model-based anchor approaches that estimate CICs using receiver operating characteristic–based and logistic regression–based analyses ranged from 2 (hemoglobin) to 8.87 (GH01) (Table 2). CIC estimates generated from the anchor-based approaches ranged from 2 to 16 (interquartile range [IQR], 5–10). Distribution-based analyses determined the CIC to be 5.62 when based on one-half standard deviation of FACIT-Fatigue at baseline, and 2.78 when based on the standard error of the measure. The empirically determined CIC for patients with CAD for FACIT-Fatigue was 5, a median estimate produced by model-based analyses that falls within the IQR of all anchor-based analyses and converges with estimates produced by distribution-based analyses.

Conclusion
This study estimated a CIC of 5 for FACIT-Fatigue in patients with CAD, a CIC consistent with estimates for other disease areas (eg, rheumatoid arthritis, systemic lupus erythematosus, anemia related to cancer). This analysis demonstrated that the FACIT-Fatigue Scale may be an appropriate and interpretable assessment of fatigue among patients with CAD.
Keyword(s): Autoimmune hemolytic anemia (AIHA), Complement, Outcome, Quality of life


