
Contributions
Abstract: EP1178
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
The lymphoma treatment landscape is changing with the introduction of chimeric antigen receptor (CAR) T cell therapies and there is a need to better understand the patient experience. During development of new therapies, patient-reported outcome measures (PROMs) provide useful information on clinical outcomes from the patient perspective. A targeted landscape review of data from published literature, clinical trial databases, and health technology assessment (HTA) reviews is a traditionally required step to identify appropriate PROMs to inform clinical development programs of new treatment options.
Aims
This landscape review aimed to identify and summarize evidence on the use of PROMs in lymphoma based on publicly available literature, product labels for medications approved by the United States Food and Drug Administration and/or the European Medicines Agency (EMA) for the treatment of lymphoma, and existing HTA information (eg, clinical effectiveness, safety, patient and social aspects) for lymphoma.
Methods
We conducted a targeted literature search using the Ovid platform and clinical trial registries from 2009–2020. English-language titles, abstracts, and clinical trial outcome measure descriptions that focused on lymphoma were reviewed, and those that mentioned the use of a PROM were deemed eligible for inclusion. Product labels were reviewed for 7 medications approved for the treatment of adult lymphoma (ie, brentuximab vedotin, lenalidomide, idelalisib, axicabtagene ciloleucel, tisagenlecleucel, pembrolizumab, pixantrone), and a review of HTA reports was carried out for non-Hodgkin lymphoma indications in 9 countries and regions over the last 5 years.
Results
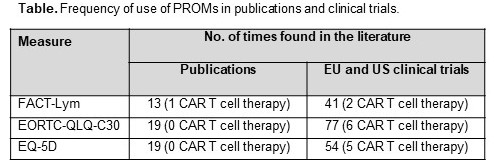
A total of 1683 articles and conference abstracts were identified and screened from the Ovid platform, of which 78 were considered eligible for inclusion. In addition, 1541 clinical trials were identified and reviewed, of which 129 reported PROM use. Among literature included, a total of 67 different PROMs were identified. The most used PROMs were Functional Assessment of Cancer Therapy – Lymphoma (FACT-Lym), European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – 30 items (EORTC QLQ-C30), and EQ-5D (See Table). The most frequently cited PROMs evaluated fatigue, pain, sleep, dermatological symptoms, and neurotoxicity. Out of 7 medications approved for adult lymphoma, brentuximab vedotin was the only one that included PROMs in its EMA labeling (EORTC QLQ-C30, EQ-5D, FACT-General); however, no clinically meaningful difference between treatment arms was reported. According to the HTA review of the 7 medications, the FACT-Lym, 36-Item Short Form Health Survey (SF-36), and Skindex-29 were all consistently accepted as relevant, validated measures to capture PRO assessments for lymphoma indications. However, treatment benefit reported by PRO data was limited by an absence of comparative data, exploratory analyses, low number of patients, and a short length of follow-up.
Conclusion
A multitude of PROMs have been employed in assessing patient burden in lymphoma. Health authorities and HTA centers recognize sensitive, validated, and reliable PROMs that report on lymphoma-specific symptom burden and patients’ health-related quality of life and well-being as critical for assessing treatment benefit and interpreting patient burden. With appropriate study design and robust PRO data collection (eg, comparative data, validated instruments), PROMs should be considered in assessing patients’ quality of life for emerging lymphoma therapies.
Keyword(s): Lymphoma, Non-Hodgkin's lymphoma, Quality of life
Abstract: EP1178
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
The lymphoma treatment landscape is changing with the introduction of chimeric antigen receptor (CAR) T cell therapies and there is a need to better understand the patient experience. During development of new therapies, patient-reported outcome measures (PROMs) provide useful information on clinical outcomes from the patient perspective. A targeted landscape review of data from published literature, clinical trial databases, and health technology assessment (HTA) reviews is a traditionally required step to identify appropriate PROMs to inform clinical development programs of new treatment options.
Aims
This landscape review aimed to identify and summarize evidence on the use of PROMs in lymphoma based on publicly available literature, product labels for medications approved by the United States Food and Drug Administration and/or the European Medicines Agency (EMA) for the treatment of lymphoma, and existing HTA information (eg, clinical effectiveness, safety, patient and social aspects) for lymphoma.
Methods
We conducted a targeted literature search using the Ovid platform and clinical trial registries from 2009–2020. English-language titles, abstracts, and clinical trial outcome measure descriptions that focused on lymphoma were reviewed, and those that mentioned the use of a PROM were deemed eligible for inclusion. Product labels were reviewed for 7 medications approved for the treatment of adult lymphoma (ie, brentuximab vedotin, lenalidomide, idelalisib, axicabtagene ciloleucel, tisagenlecleucel, pembrolizumab, pixantrone), and a review of HTA reports was carried out for non-Hodgkin lymphoma indications in 9 countries and regions over the last 5 years.
Results
A total of 1683 articles and conference abstracts were identified and screened from the Ovid platform, of which 78 were considered eligible for inclusion. In addition, 1541 clinical trials were identified and reviewed, of which 129 reported PROM use. Among literature included, a total of 67 different PROMs were identified. The most used PROMs were Functional Assessment of Cancer Therapy – Lymphoma (FACT-Lym), European Organization for Research and Treatment of Cancer Quality of Life Questionnaire – 30 items (EORTC QLQ-C30), and EQ-5D (See Table). The most frequently cited PROMs evaluated fatigue, pain, sleep, dermatological symptoms, and neurotoxicity. Out of 7 medications approved for adult lymphoma, brentuximab vedotin was the only one that included PROMs in its EMA labeling (EORTC QLQ-C30, EQ-5D, FACT-General); however, no clinically meaningful difference between treatment arms was reported. According to the HTA review of the 7 medications, the FACT-Lym, 36-Item Short Form Health Survey (SF-36), and Skindex-29 were all consistently accepted as relevant, validated measures to capture PRO assessments for lymphoma indications. However, treatment benefit reported by PRO data was limited by an absence of comparative data, exploratory analyses, low number of patients, and a short length of follow-up.

Conclusion
A multitude of PROMs have been employed in assessing patient burden in lymphoma. Health authorities and HTA centers recognize sensitive, validated, and reliable PROMs that report on lymphoma-specific symptom burden and patients’ health-related quality of life and well-being as critical for assessing treatment benefit and interpreting patient burden. With appropriate study design and robust PRO data collection (eg, comparative data, validated instruments), PROMs should be considered in assessing patients’ quality of life for emerging lymphoma therapies.
Keyword(s): Lymphoma, Non-Hodgkin's lymphoma, Quality of life


