
Contributions
Abstract: EP1163
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
The phase 1 portion of the phase 1/2 trial of REGN5458, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, demonstrated an acceptable safety and tolerability profile with early, deep and durable efficacy in heavily pre-treated patients with RRMM (NCT03761108).1
Aims
To report the effects of REGN5458 on patient-reported outcomes (PROs).
Methods
Patients with progressive RRMM (≥3 prior lines of systemic therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti‑CD38 antibody) were enrolled in the study. Patients received weekly doses of REGN5458, followed by continuous dosing, administered every 2 weeks. A modified 3+3 dose escalation design (4+3) was used. PROs were assessed using multiple instruments including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30). Analyses were performed on patients (N=49) who received REGN5458, pooled across dose levels 1 to 6, at baseline and Weeks 4, 8, 12 and 24. These included A) descriptive mean scores at each assessment, B) change from baseline scores over time and C) estimated overall change from baseline using mixed-effects model for repeated measures analysis [and 95% confidence intervals (CI)] in the global health status/quality of life (GHS/QoL), and all functional and symptom scales from the EORTC QLQ-C30. No multiplicity adjustments were made, and findings are exploratory. Clinically meaningful change was defined as a ≥10-point change from baseline, statistical significance was interpreted using 95% CIs of change from the baseline.
Results
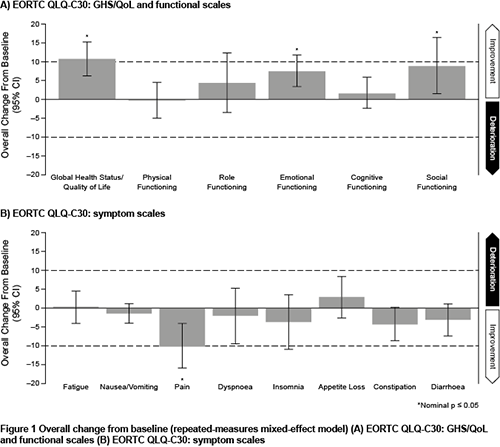
At baseline, patients had a median of five prior lines of therapy. Statistically significant and clinically meaningful overall improvements from baseline in GHS/QoL (10.7 [95% CI: 6.2, 15.1]) and patient-reported pain symptoms (-10.2 [95% CI: -16.2, -4.2]) were observed with REGN5458. Statistically significant overall improvements from baseline in emotional functioning (7.6 [95% CI: 3.4, 11.7]) and social functioning (8.9 [95% CI: 1.6, 16.3]) were also seen. Across remaining EORTC QLQ-C30 functional (Figure 1A) and symptom (Figure 1B) scales, improved trends were observed, with the exception of physical functioning (-0.2 [95% CI: -5.0, 4.6]), fatigue (0.3 [95% CI: -4.0, 4.6]), and appetite loss (3.0 [95% CI: -2.6, 8.5]).
Conclusion
Patients who received REGN5458 showed statistically significant and clinically meaningful overall improvements in GHS/QoL and pain symptoms. Furthermore, there was no statistically significant overall deterioration in PROs even in a heavily pre-treated population. Additional and longer follow-up PRO data are warranted to confirm the benefit-risk profile of REGN5458 in heavily pre-treated patients with RRMM.
1. Madduri D et al. ASH 2020. O291
Keyword(s): B-cell maturation antigen, Bispecific, Multiple myeloma, Quality of life
Abstract: EP1163
Type: E-Poster Presentation
Session title: Quality of life, palliative care, ethics and health economics
Background
The phase 1 portion of the phase 1/2 trial of REGN5458, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, demonstrated an acceptable safety and tolerability profile with early, deep and durable efficacy in heavily pre-treated patients with RRMM (NCT03761108).1
Aims
To report the effects of REGN5458 on patient-reported outcomes (PROs).
Methods
Patients with progressive RRMM (≥3 prior lines of systemic therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti‑CD38 antibody) were enrolled in the study. Patients received weekly doses of REGN5458, followed by continuous dosing, administered every 2 weeks. A modified 3+3 dose escalation design (4+3) was used. PROs were assessed using multiple instruments including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30). Analyses were performed on patients (N=49) who received REGN5458, pooled across dose levels 1 to 6, at baseline and Weeks 4, 8, 12 and 24. These included A) descriptive mean scores at each assessment, B) change from baseline scores over time and C) estimated overall change from baseline using mixed-effects model for repeated measures analysis [and 95% confidence intervals (CI)] in the global health status/quality of life (GHS/QoL), and all functional and symptom scales from the EORTC QLQ-C30. No multiplicity adjustments were made, and findings are exploratory. Clinically meaningful change was defined as a ≥10-point change from baseline, statistical significance was interpreted using 95% CIs of change from the baseline.
Results
At baseline, patients had a median of five prior lines of therapy. Statistically significant and clinically meaningful overall improvements from baseline in GHS/QoL (10.7 [95% CI: 6.2, 15.1]) and patient-reported pain symptoms (-10.2 [95% CI: -16.2, -4.2]) were observed with REGN5458. Statistically significant overall improvements from baseline in emotional functioning (7.6 [95% CI: 3.4, 11.7]) and social functioning (8.9 [95% CI: 1.6, 16.3]) were also seen. Across remaining EORTC QLQ-C30 functional (Figure 1A) and symptom (Figure 1B) scales, improved trends were observed, with the exception of physical functioning (-0.2 [95% CI: -5.0, 4.6]), fatigue (0.3 [95% CI: -4.0, 4.6]), and appetite loss (3.0 [95% CI: -2.6, 8.5]).

Conclusion
Patients who received REGN5458 showed statistically significant and clinically meaningful overall improvements in GHS/QoL and pain symptoms. Furthermore, there was no statistically significant overall deterioration in PROs even in a heavily pre-treated population. Additional and longer follow-up PRO data are warranted to confirm the benefit-risk profile of REGN5458 in heavily pre-treated patients with RRMM.
1. Madduri D et al. ASH 2020. O291
Keyword(s): B-cell maturation antigen, Bispecific, Multiple myeloma, Quality of life


