
Contributions
Abstract: EP1157
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is a rare autoimmune disease characterised by low platelet counts. Romiplostim is a thrombopoietin-receptor agonist (TPO-RA) that increases platelet production and is indicated in Europe for the treatment of primary ITP in adult patients (pts) who are refractory to other treatments (e.g. corticosteroids/immunoglobulins). In 2021, romiplostim was approved for use in all phases of ITP, i.e. newly diagnosed (0–3 months [M]), persistent (>3–12M) and chronic ITP (>12M). This is in line with current European guidelines to switch adult pts earlier from corticosteroids to TPO-RAs. Although romiplostim was previously restricted to chronic ITP, there has been clinical evidence supporting the use of romiplostim in earlier disease phases (Kuter, Br J Haematol 2019; Newland, Br J Haematol 2016). Real-world data on the routine use of romiplostim in pts with ITP duration of ≤12M can help guide current and future clinical practice.
Aims
To assess the effectiveness and safety of romiplostim in adults with primary ITP, according to ITP phase, in routine clinical practice in Germany.
Methods
This was a post-hoc analysis of the single-arm, prospective, observational PLATEAU study. Data were collected from adult pts (≥18 years of age) who received ≥1 dose of romiplostim in haemato-oncological centres across Germany during the years 2010–2016. After the first romiplostim dose, follow-up data were collected for up to 2 years or until loss to follow up/death. Retrospective data were collected for bleeding events for up to 6M prior to romiplostim. Overall platelet response was defined as ≥1 platelet count measurement ≥50 x 109/L during weeks 2–24 after romiplostim initiation. Durable platelet response was achieved if ≥75% of all platelet count measurements were ≥50 x 109/L during weeks 14–24 after romiplostim initiation. Other outcomes included patient demographics, romiplostim use, and adverse drug reactions (ADRs). Subgroup analyses were performed according to ITP phases (time from diagnosis to romiplostim initiation: <3M; 3–12M; >12M).
Results
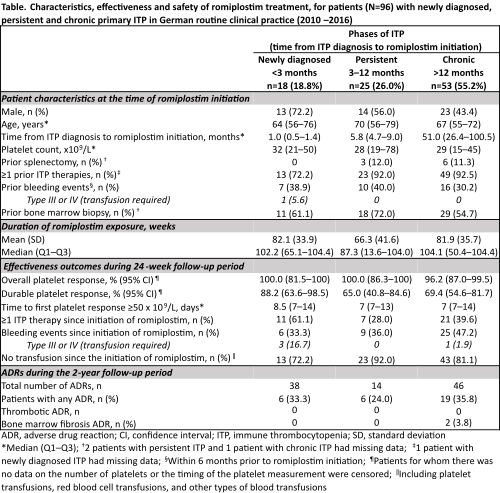
A total of 96 pts were included in the analysis. At the time of romiplostim initiation, 18 (18.8%) were newly diagnosed, 25 (26.0%) had persistent ITP, and 53 (55.2%) had chronic ITP (Table). The median (Q1–Q3) time from ITP diagnosis to romiplostim initiation was 14.9M (4.3–54.2). The proportion of pts who were non-splenectomised at treatment initiation was 87.5%. No newly diagnosed pts were splenectomised (versus 12.0% and 11.3% of pts with persistent or chronic ITP, respectively). Overall platelet response was achieved in 100% of pts with newly diagnosed and persistent ITP, and in 96.2% of pts with chronic ITP. Durable platelet response was achieved in 88.2% of newly diagnosed pts, 65.0% of pts with persistent ITP, and 69.4% of pts with chronic ITP. The median time to first platelet response was 8.5 days (newly diagnosed), and 7 days (for both persistent and chronic ITP). Safety was comparable across all ITP phases; there were two occurrences of bone marrow ADRs, both in pts with chronic ITP, and no thrombotic ADRs.
Conclusion
In this observational study, clinically meaningful platelet responses were achieved by romiplostim-treated pts in all ITP phases. Romiplostim was well tolerated. This cohort, representative of routine clinical practice in Germany, supports the effectiveness and safety of romiplostim in pts with newly diagnosed and persistent ITP in routine clinical practice, in line with recent guidelines.
Keyword(s): Immune thrombocytopenia (ITP), ITP, Platelet, TPO
Abstract: EP1157
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenia (ITP) is a rare autoimmune disease characterised by low platelet counts. Romiplostim is a thrombopoietin-receptor agonist (TPO-RA) that increases platelet production and is indicated in Europe for the treatment of primary ITP in adult patients (pts) who are refractory to other treatments (e.g. corticosteroids/immunoglobulins). In 2021, romiplostim was approved for use in all phases of ITP, i.e. newly diagnosed (0–3 months [M]), persistent (>3–12M) and chronic ITP (>12M). This is in line with current European guidelines to switch adult pts earlier from corticosteroids to TPO-RAs. Although romiplostim was previously restricted to chronic ITP, there has been clinical evidence supporting the use of romiplostim in earlier disease phases (Kuter, Br J Haematol 2019; Newland, Br J Haematol 2016). Real-world data on the routine use of romiplostim in pts with ITP duration of ≤12M can help guide current and future clinical practice.
Aims
To assess the effectiveness and safety of romiplostim in adults with primary ITP, according to ITP phase, in routine clinical practice in Germany.
Methods
This was a post-hoc analysis of the single-arm, prospective, observational PLATEAU study. Data were collected from adult pts (≥18 years of age) who received ≥1 dose of romiplostim in haemato-oncological centres across Germany during the years 2010–2016. After the first romiplostim dose, follow-up data were collected for up to 2 years or until loss to follow up/death. Retrospective data were collected for bleeding events for up to 6M prior to romiplostim. Overall platelet response was defined as ≥1 platelet count measurement ≥50 x 109/L during weeks 2–24 after romiplostim initiation. Durable platelet response was achieved if ≥75% of all platelet count measurements were ≥50 x 109/L during weeks 14–24 after romiplostim initiation. Other outcomes included patient demographics, romiplostim use, and adverse drug reactions (ADRs). Subgroup analyses were performed according to ITP phases (time from diagnosis to romiplostim initiation: <3M; 3–12M; >12M).
Results
A total of 96 pts were included in the analysis. At the time of romiplostim initiation, 18 (18.8%) were newly diagnosed, 25 (26.0%) had persistent ITP, and 53 (55.2%) had chronic ITP (Table). The median (Q1–Q3) time from ITP diagnosis to romiplostim initiation was 14.9M (4.3–54.2). The proportion of pts who were non-splenectomised at treatment initiation was 87.5%. No newly diagnosed pts were splenectomised (versus 12.0% and 11.3% of pts with persistent or chronic ITP, respectively). Overall platelet response was achieved in 100% of pts with newly diagnosed and persistent ITP, and in 96.2% of pts with chronic ITP. Durable platelet response was achieved in 88.2% of newly diagnosed pts, 65.0% of pts with persistent ITP, and 69.4% of pts with chronic ITP. The median time to first platelet response was 8.5 days (newly diagnosed), and 7 days (for both persistent and chronic ITP). Safety was comparable across all ITP phases; there were two occurrences of bone marrow ADRs, both in pts with chronic ITP, and no thrombotic ADRs.

Conclusion
In this observational study, clinically meaningful platelet responses were achieved by romiplostim-treated pts in all ITP phases. Romiplostim was well tolerated. This cohort, representative of routine clinical practice in Germany, supports the effectiveness and safety of romiplostim in pts with newly diagnosed and persistent ITP in routine clinical practice, in line with recent guidelines.
Keyword(s): Immune thrombocytopenia (ITP), ITP, Platelet, TPO


