Contributions
Abstract: EP1151
Type: E-Poster Presentation
Session title: Platelet disorders
Background
The recent ASH Guidelines1 and International Consensus Report2 for the management of ITP suggest consideration of TPO-RAs as subsequent therapy following an insufficient response to first-line treatments, especially if seeking a durable platelet response. Avatrombopag (AVA), a TPO-RA which was FDA approved in 2019, also recently received EMA approval for adults suffering with chronic ITP. AVA is orally-administered, does not have food type restrictions, nor carry a boxed warning for hepatoxicity or requires liver function monitoring.
Aims
To understand length of therapy (LOT) and persistence (PER) of treatment in adult ITP patients in US, treated with an FDA-approved TPO-RA (AVA, eltrombopag (ELT) or romiplostim (ROMI).
Methods
The Symphony Health Claims Database3 was used to understand LOT and PER for ELT and ROMI in US adult ITP patients. Claims data were evaluated and inclusion in the analyses required a D69.3 or D69.49 ICD-10 diagnostic code for ITP, and ≥ 1 first-initiated claim for ELT or ROMI during the 2019 calendar year. Due to AVA claims not being available in the Symphony Database, data from a closed system of specialty pharmacies (CVS, Accredo, Biologics, Kroger, PharmaCord, Panther) accounting for ~78% of AVA prescriptions were used. Adult patients with a D69.3 or D69.49 diagnostic code, receiving ≥ 1 initial shipment of AVA from August 2019 through December 2019 were included in the evaluation, with LOT and PER calculated over the first 365 days following initial shipment of AVA from the specialty pharmacy. Days of supply were considered to be 7 days per administration for ROMI and were provided specifically in each claim for ELT, with no cap on supply. Days of supply for AVA were provided by the pharmacy with a maximum of 90 days per shipment.
LOT for each TPO-RA was determined by calculating the time between the first claim/shipment date and the last claim/shipment date, while adding on the number of days of supply provided in the last claim/shipment (e.g. 30 day supply).
PER for each TPO-RA were determined by claims occurring in specific months (e.g. a patient with a claim on January 31st and another on February 1st would have 2 months of persistence). For each therapy, gaps in claims/shipments were allowed, with no max duration between data points. Unlike LOT, PER did not add the number of days of supply to the last evaluable claim/shipment.
Results
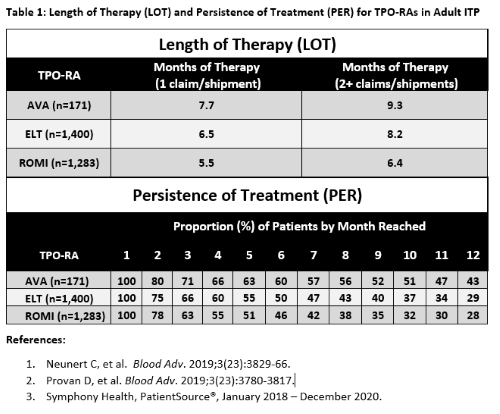
LOT and PER for AVA, ELT and ROMI are shown below (Table 1). 171 unique AVA patients were captured via specialty pharmacy data, while 1,400 ELT and 1,283 ROMI patients had evaluable claims. LOT was highest in AVA patients, regardless of whether a conservative single claim/shipment, or ≥ 2 claims/shipments were used for inclusion, with a max of 9.3 months of therapy administered over a potential max 12-month time frame. PER for each individual month reached was highest for AVA, with the median AVA patient reaching 10 months of treatment.
Conclusion
These data suggest that AVA has greater length of treatment and higher rates of persistence than other TPO-RAs, which may be due to a variety of variables including 1) route of administration, 2) convenience, 3) patient preference, 4) efficacy, 5) side effects, 6) insurance coverage. These results should be interpreted with caution. Claims data using matched methodologies were utilized to calculate LOT and PER for each TPO-RA, however due to AVA’s commercial availability occurring in July 2019, slightly different time frames were evaluated. Also, different sources of data capture were necessary due to lack of a consistent source.
Keyword(s):
Abstract: EP1151
Type: E-Poster Presentation
Session title: Platelet disorders
Background
The recent ASH Guidelines1 and International Consensus Report2 for the management of ITP suggest consideration of TPO-RAs as subsequent therapy following an insufficient response to first-line treatments, especially if seeking a durable platelet response. Avatrombopag (AVA), a TPO-RA which was FDA approved in 2019, also recently received EMA approval for adults suffering with chronic ITP. AVA is orally-administered, does not have food type restrictions, nor carry a boxed warning for hepatoxicity or requires liver function monitoring.
Aims
To understand length of therapy (LOT) and persistence (PER) of treatment in adult ITP patients in US, treated with an FDA-approved TPO-RA (AVA, eltrombopag (ELT) or romiplostim (ROMI).
Methods
The Symphony Health Claims Database3 was used to understand LOT and PER for ELT and ROMI in US adult ITP patients. Claims data were evaluated and inclusion in the analyses required a D69.3 or D69.49 ICD-10 diagnostic code for ITP, and ≥ 1 first-initiated claim for ELT or ROMI during the 2019 calendar year. Due to AVA claims not being available in the Symphony Database, data from a closed system of specialty pharmacies (CVS, Accredo, Biologics, Kroger, PharmaCord, Panther) accounting for ~78% of AVA prescriptions were used. Adult patients with a D69.3 or D69.49 diagnostic code, receiving ≥ 1 initial shipment of AVA from August 2019 through December 2019 were included in the evaluation, with LOT and PER calculated over the first 365 days following initial shipment of AVA from the specialty pharmacy. Days of supply were considered to be 7 days per administration for ROMI and were provided specifically in each claim for ELT, with no cap on supply. Days of supply for AVA were provided by the pharmacy with a maximum of 90 days per shipment.
LOT for each TPO-RA was determined by calculating the time between the first claim/shipment date and the last claim/shipment date, while adding on the number of days of supply provided in the last claim/shipment (e.g. 30 day supply).
PER for each TPO-RA were determined by claims occurring in specific months (e.g. a patient with a claim on January 31st and another on February 1st would have 2 months of persistence). For each therapy, gaps in claims/shipments were allowed, with no max duration between data points. Unlike LOT, PER did not add the number of days of supply to the last evaluable claim/shipment.
Results
LOT and PER for AVA, ELT and ROMI are shown below (Table 1). 171 unique AVA patients were captured via specialty pharmacy data, while 1,400 ELT and 1,283 ROMI patients had evaluable claims. LOT was highest in AVA patients, regardless of whether a conservative single claim/shipment, or ≥ 2 claims/shipments were used for inclusion, with a max of 9.3 months of therapy administered over a potential max 12-month time frame. PER for each individual month reached was highest for AVA, with the median AVA patient reaching 10 months of treatment.
Conclusion
These data suggest that AVA has greater length of treatment and higher rates of persistence than other TPO-RAs, which may be due to a variety of variables including 1) route of administration, 2) convenience, 3) patient preference, 4) efficacy, 5) side effects, 6) insurance coverage. These results should be interpreted with caution. Claims data using matched methodologies were utilized to calculate LOT and PER for each TPO-RA, however due to AVA’s commercial availability occurring in July 2019, slightly different time frames were evaluated. Also, different sources of data capture were necessary due to lack of a consistent source.
Keyword(s):