Contributions
Abstract: EP1148
Type: E-Poster Presentation
Session title: Platelet disorders
Background
The clinical management of ITP has been evolving. Thrombopoietin receptor agonists (TPO-RAs) have become widely utilized as second-line treatments, and the 2019 ASH guidelines recommend their use over rituximab to achieve a durable response. Avatrombopag (AVA) is an oral TPO-RA approved in 2019 for patients (Pts) with ITP. A high proportion of Pts (~90%) respond to AVA; but limited information is available regarding the durability of response over time utilizing clinically relevant loss of response (LOR) definitions.
Aims
To understand the time it took Pts to experience their first LOR and the overall percent of treatment days a response level platelet (PC) was achieved.
Methods
A Phase 3 study (NCT01438840) enrolled 32 AVA and 17 placebo-treated (PBO) Pts with ITP. The core study design included a 6-week study drug titration period, 12-week concomitant ITP medication reduction period, and an 8-week maintenance period. Subjects who completed the maintenance phase of the core or discontinued early because of lack of treatment effect were eligible for the Extension Phase. These Pts all received open-label 20 mg AVA once daily and underwent a 6-week conversion period, and a 90-week maintenance period.
For this post-hoc analysis, we assessed how many days it took for Pts randomized to AVA who responded in the Core and continued into the extension to experience their first LOR or reach study conclusion. After the initial PC ≥50,000/µL was noted, we also examined the percentage of remaining treatment time that initial response was maintained.
Response was defined as the first time achieving a PC ≥50,000/µL. LOR was defined as a Pt experiencing a PC <30,000/µL for 4 consecutive weeks (LOR-4wk), or in a more conservative manner, Pts experiencing a PC <30,000/µL on 2 consecutive scheduled visits (LOR-2vis). During the Core, visits were weekly or biweekly depending on the phase of the study with 21 visits occurring over 26 weeks. In the Extension, visits were every 3-4 days, weekly, bi-weekly, or monthly depending on the phase with 31 planned over 96 weeks. The protocol was amended to end the study on 3/10/2014 when the last patient exited the Core Study.
When a LOR was observed, the date of the first PC <30,000/µL was used for subsequent calculations with a return of response defined as a PC exceeding ≥30,000/µL again. Pts who required rescue therapy (n=4) were included in the analyses and the first date of administration was used for the LOR date. Pts requiring corticosteroids (n=3) as rescue therapy were considered non-responders for a minimum of 8 weeks and Pts receiving a platelet transfusion (n=1) for a minimum of 1 week.
Results
32 Pts were randomized to AVA, 29 responded, and 21 continued into the Extension Phase and were evaluated here.
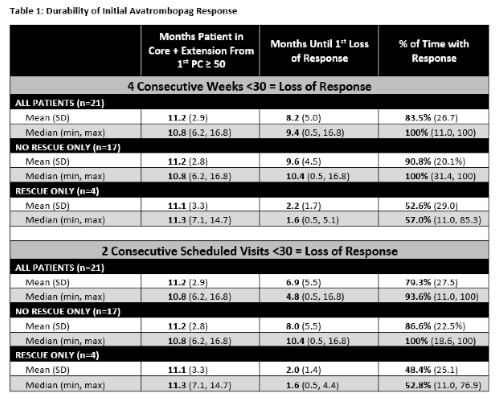
61.9% (13/21) and 42.9% (9/21) of AVA-responsive Pts never experienced a LOR-4wk or LOR-2vis, respectively. The average number of months until all Pts experienced their first LOR-4wk, discontinued, or completed the study was 8.2 months (median 9.4) and 6.9 months (median 4.8) for the LOR-2vis analysis. Pts maintained their initial response on average for 83.5% (median 100%) of their remaining time in the study for LOR-4wk and for 79.3% (median 100%) in the LOR-2vis analyses (Table 1).
Conclusion
These data suggest that the initial response to AVA is durable, with up to 62% of Pts never experiencing a LOR in the core or extension, and stable with Pts maintaining a response on average for up to 84% of the time despite the planned objective of reducing concomitant medications and required AVA starting dose in the extension.
Keyword(s): Immune thrombocytopenia (ITP), Platelet count
Abstract: EP1148
Type: E-Poster Presentation
Session title: Platelet disorders
Background
The clinical management of ITP has been evolving. Thrombopoietin receptor agonists (TPO-RAs) have become widely utilized as second-line treatments, and the 2019 ASH guidelines recommend their use over rituximab to achieve a durable response. Avatrombopag (AVA) is an oral TPO-RA approved in 2019 for patients (Pts) with ITP. A high proportion of Pts (~90%) respond to AVA; but limited information is available regarding the durability of response over time utilizing clinically relevant loss of response (LOR) definitions.
Aims
To understand the time it took Pts to experience their first LOR and the overall percent of treatment days a response level platelet (PC) was achieved.
Methods
A Phase 3 study (NCT01438840) enrolled 32 AVA and 17 placebo-treated (PBO) Pts with ITP. The core study design included a 6-week study drug titration period, 12-week concomitant ITP medication reduction period, and an 8-week maintenance period. Subjects who completed the maintenance phase of the core or discontinued early because of lack of treatment effect were eligible for the Extension Phase. These Pts all received open-label 20 mg AVA once daily and underwent a 6-week conversion period, and a 90-week maintenance period.
For this post-hoc analysis, we assessed how many days it took for Pts randomized to AVA who responded in the Core and continued into the extension to experience their first LOR or reach study conclusion. After the initial PC ≥50,000/µL was noted, we also examined the percentage of remaining treatment time that initial response was maintained.
Response was defined as the first time achieving a PC ≥50,000/µL. LOR was defined as a Pt experiencing a PC <30,000/µL for 4 consecutive weeks (LOR-4wk), or in a more conservative manner, Pts experiencing a PC <30,000/µL on 2 consecutive scheduled visits (LOR-2vis). During the Core, visits were weekly or biweekly depending on the phase of the study with 21 visits occurring over 26 weeks. In the Extension, visits were every 3-4 days, weekly, bi-weekly, or monthly depending on the phase with 31 planned over 96 weeks. The protocol was amended to end the study on 3/10/2014 when the last patient exited the Core Study.
When a LOR was observed, the date of the first PC <30,000/µL was used for subsequent calculations with a return of response defined as a PC exceeding ≥30,000/µL again. Pts who required rescue therapy (n=4) were included in the analyses and the first date of administration was used for the LOR date. Pts requiring corticosteroids (n=3) as rescue therapy were considered non-responders for a minimum of 8 weeks and Pts receiving a platelet transfusion (n=1) for a minimum of 1 week.
Results
32 Pts were randomized to AVA, 29 responded, and 21 continued into the Extension Phase and were evaluated here.
61.9% (13/21) and 42.9% (9/21) of AVA-responsive Pts never experienced a LOR-4wk or LOR-2vis, respectively. The average number of months until all Pts experienced their first LOR-4wk, discontinued, or completed the study was 8.2 months (median 9.4) and 6.9 months (median 4.8) for the LOR-2vis analysis. Pts maintained their initial response on average for 83.5% (median 100%) of their remaining time in the study for LOR-4wk and for 79.3% (median 100%) in the LOR-2vis analyses (Table 1).
Conclusion
These data suggest that the initial response to AVA is durable, with up to 62% of Pts never experiencing a LOR in the core or extension, and stable with Pts maintaining a response on average for up to 84% of the time despite the planned objective of reducing concomitant medications and required AVA starting dose in the extension.
Keyword(s): Immune thrombocytopenia (ITP), Platelet count