
Contributions
Abstract: EP1144
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Avatrombopag (AVA) is an oral thrombopoietin receptor agonist (TPO-RA) approved for treatment of chronic immune thrombocytopenia (ITP). Data describing effectiveness of AVA following treatment with other TPO-RAs is limited.
Aims
Evaluate ITP treatment outcomes in patients who switched from romiplostim or eltrombopag (ROMI/ELT) to AVA.
Methods
We retrospectively evaluated all adults with ITP switched from ROMI/ELT to AVA at four U.S. tertiary ITP referral centers from July 2019 through December 2020 who were treated with AVA for at least two months by the data cutoff date.
Results
Patient Characteristics: 45 patients were included, with a median (range) age of 60 (21-87) years; 53% were female. Mean treatment duration with ROMI/ELT prior to switch was 26.4 months and mean AVA treatment duration was 9 months. At AVA initiation, patients had ITP for a mean of 8.3 years with a mean (range) of 4.8 (2-10) unique prior ITP treatments. The reason for switching to AVA was convenience in 23 patients (51%), ineffectiveness of ROMI/ELT in 14 (31%), and adverse event on ROMI/ELT in 8 (18%). The TPO-RA prior to switch was ROMI in 33 patients (73%), ELT in 11 (24%) and both simultaneously in 1 (2%).
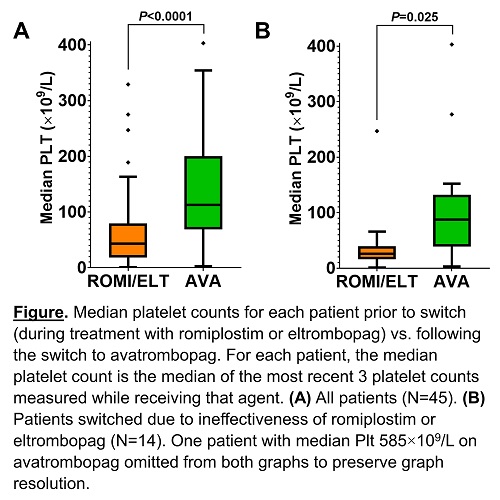
Platelet Outcomes: In all patients, the median platelet count (Plt) on ROMI/ELT was 45×109/L vs. 114×109/L on AVA (P<0.0001); in patients switched for ineffectiveness of ROMI/ELT, it was 28×109/L on ROMI/ELT vs. 88×109/L on AVA (P=0.025), FIGURE. On AVA, in the absence of rescue therapy (>8 weeks from rescue corticosteroids or >4 weeks from rescue IVIG), Plt ≥50×109/L was achieved by 42/45 patients (93%), including 12/14 (86%) switched due to ineffectiveness of ROMI/ELT; Plt ≥100×109/L (complete response) was achieved by 39/45 patients (87%), including 10/14 (71%) switched due to ineffectiveness of ROMI/ELT.
AVA Dosing: The median total weekly AVA dose was 140 mg (20 mg daily) in all patients and 280 mg (40 mg daily) in patients switching due to ROMI/ELT ineffectiveness.
Concomitant Medications/Rescue Therapy: Of the 19 patients who required concomitant corticosteroids while on ROMI/ELT, 12 (63%) were able to discontinue steroids, 6 (32%) reduced steroid dose, and none increased steroid dose after switching to AVA. 15 patients (33%) required rescue in the year prior to switching versus 9 (20%) following the switch; this included 4 patients who had also required rescue on ROMI/ELT and 5 who had not previously required rescue. 11 patients requiring rescue on ROMI/ELT did not require rescue after the switch to AVA.
AVA Discontinuation: 7 patients (16%) discontinued AVA, including 2 patients (4%) for adverse events (headache, portal vein thrombosis), 1 (2%) for lack of response, 1 for patient preference, 1 for achievement of remission, 1 for transition to rituximab to treat concomitant hemolytic anemia (patient with Evans syndrome), and 1 due to lack of the medication on a formulary.
Conclusion
In a heavily pretreated chronic ITP population, AVA was effective following therapy with ROMI/ELT, with high response rates even in patients with inadequate response to a prior TPO-RA.
Keyword(s): Clinical outcome, Immune thrombocytopenia (ITP), Thrombopoietin (TPO)
Abstract: EP1144
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Avatrombopag (AVA) is an oral thrombopoietin receptor agonist (TPO-RA) approved for treatment of chronic immune thrombocytopenia (ITP). Data describing effectiveness of AVA following treatment with other TPO-RAs is limited.
Aims
Evaluate ITP treatment outcomes in patients who switched from romiplostim or eltrombopag (ROMI/ELT) to AVA.
Methods
We retrospectively evaluated all adults with ITP switched from ROMI/ELT to AVA at four U.S. tertiary ITP referral centers from July 2019 through December 2020 who were treated with AVA for at least two months by the data cutoff date.
Results
Patient Characteristics: 45 patients were included, with a median (range) age of 60 (21-87) years; 53% were female. Mean treatment duration with ROMI/ELT prior to switch was 26.4 months and mean AVA treatment duration was 9 months. At AVA initiation, patients had ITP for a mean of 8.3 years with a mean (range) of 4.8 (2-10) unique prior ITP treatments. The reason for switching to AVA was convenience in 23 patients (51%), ineffectiveness of ROMI/ELT in 14 (31%), and adverse event on ROMI/ELT in 8 (18%). The TPO-RA prior to switch was ROMI in 33 patients (73%), ELT in 11 (24%) and both simultaneously in 1 (2%).
Platelet Outcomes: In all patients, the median platelet count (Plt) on ROMI/ELT was 45×109/L vs. 114×109/L on AVA (P<0.0001); in patients switched for ineffectiveness of ROMI/ELT, it was 28×109/L on ROMI/ELT vs. 88×109/L on AVA (P=0.025), FIGURE. On AVA, in the absence of rescue therapy (>8 weeks from rescue corticosteroids or >4 weeks from rescue IVIG), Plt ≥50×109/L was achieved by 42/45 patients (93%), including 12/14 (86%) switched due to ineffectiveness of ROMI/ELT; Plt ≥100×109/L (complete response) was achieved by 39/45 patients (87%), including 10/14 (71%) switched due to ineffectiveness of ROMI/ELT.
AVA Dosing: The median total weekly AVA dose was 140 mg (20 mg daily) in all patients and 280 mg (40 mg daily) in patients switching due to ROMI/ELT ineffectiveness.
Concomitant Medications/Rescue Therapy: Of the 19 patients who required concomitant corticosteroids while on ROMI/ELT, 12 (63%) were able to discontinue steroids, 6 (32%) reduced steroid dose, and none increased steroid dose after switching to AVA. 15 patients (33%) required rescue in the year prior to switching versus 9 (20%) following the switch; this included 4 patients who had also required rescue on ROMI/ELT and 5 who had not previously required rescue. 11 patients requiring rescue on ROMI/ELT did not require rescue after the switch to AVA.
AVA Discontinuation: 7 patients (16%) discontinued AVA, including 2 patients (4%) for adverse events (headache, portal vein thrombosis), 1 (2%) for lack of response, 1 for patient preference, 1 for achievement of remission, 1 for transition to rituximab to treat concomitant hemolytic anemia (patient with Evans syndrome), and 1 due to lack of the medication on a formulary.

Conclusion
In a heavily pretreated chronic ITP population, AVA was effective following therapy with ROMI/ELT, with high response rates even in patients with inadequate response to a prior TPO-RA.
Keyword(s): Clinical outcome, Immune thrombocytopenia (ITP), Thrombopoietin (TPO)


