
Contributions
Abstract: EP1140
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenic purpura (ITP) is an immune mediated acquired disorder characterized by decreased platelet count and increased risk of bleeding. The disease is classified into 3 categories according to the time from diagnosis: newly-diagnosed (within 3 months of onset), persistent (between 3 and 12 months), chronic (beyond 12 months). Eltrombopag (EPAG) is an orally TPO-RA (Thrombopoietin-Receptor Agonist), approved for patients with pediatric chronic ITP older than 1 year, refractory to first line therapy. In two Randomized Controlled Trials conducted in pediatric age, EPAG led to an improvement of platelet counts and a reduction in bleeding severity. In addition, in some studies conducted on adults with newly-diagnosed ITP the use off-label of EPAG has also shown good results. However, there is still a significant proportion (about 20%) of pediatric patients who do not show a response to EPAG. Scarce data are currently available regarding the pharmacokinetics of EPAG in children: the estimated median plasma EPAG time to maximum plasma concentration (Tmax) and geometric mean plasma EPAG half-life (t1/2) values are consistent with the adult values, respectively 4 hours and 47-51 hours. The area under plasma concentration-time curve over a dosing interval at steady-state (AUC(0-t)) and maximum plasma concentration (Cmax) values were similar between adolescents and adults while children have higher dose normalized AUC(0-t) and Cmax.
Aims
Explore potential variability of EPAG pharmacokinetic in a cohort of pediatric patients affected by refractory ITP with no or variable response to EPAG or who experienced significant side effects.
Methods
Subjects aged 0 to 18 years were enrolled for pharmacokinetic assessment. Blood samples were obtained at 0, 2, 4 and 8 hours after the administration. Patients had started EPAG at least since 2 weeks. Analysis of EPAG plasma concentration was performed by mass spectrometry coupled with UHPLC (Ultra High Performance Liquid Chromatography) platform.
Results
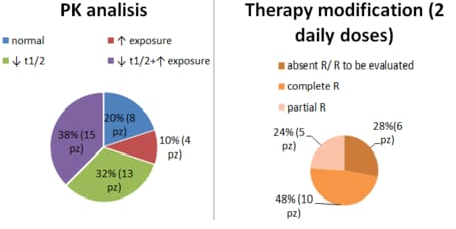
We performed 40 PK analysis (24 Females, 16 Males). Patients who started therapy at different stages of the disease were considered (16 newly diagnosed, 12 persistent, 12 chronic). The median dose of EPAG given once daily was 50 mg, ranging from 12.5 mg (n subjects=3) to 150 mg (n subject=1). Overall, EPAG peak (Tmax) occured between 2 and 4 hours with a population Cmax of 50 ug/mL and t1/2 of 25 hours. Our cohort of patients therefore showed a Tmax consistent with the literature values, on the other hand, t1/2 appeared to be reduced when compared to known data. Moreover, 32% (13 patients) of our population had an extremely short half-life with values below 12 hours. With regard to exposure, 47.5% of patients (19 patients) have higher Cmax and AUC(0-t) values compared to literature. Based on these results, we tried to change the time of administration of EPAG in about 21 patients, who had shortened t1/2 and/or increased exposure, dividing the daily dose into 2 administrations. 15 patients showed a response, 10 showed a complete response (PLTs >100.000), 5 a partial response (PLTs 30.000-100.000), although 2 patients did not maintain the response in the follow-up and 1 had a fluctuating response.
Conclusion
These data highlight the need to further explore variability of EPAG exposure and its pharmacokinetic/pharmacodynamic profile in the pediatric population. Our results also suggest the possibility of improving the response to EPAG by modifying the administration although further studies are needed.
Keyword(s): Pediatric, Pharmacokinetic, Thrombocytopenia, Thrombopoietin (TPO)
Abstract: EP1140
Type: E-Poster Presentation
Session title: Platelet disorders
Background
Immune thrombocytopenic purpura (ITP) is an immune mediated acquired disorder characterized by decreased platelet count and increased risk of bleeding. The disease is classified into 3 categories according to the time from diagnosis: newly-diagnosed (within 3 months of onset), persistent (between 3 and 12 months), chronic (beyond 12 months). Eltrombopag (EPAG) is an orally TPO-RA (Thrombopoietin-Receptor Agonist), approved for patients with pediatric chronic ITP older than 1 year, refractory to first line therapy. In two Randomized Controlled Trials conducted in pediatric age, EPAG led to an improvement of platelet counts and a reduction in bleeding severity. In addition, in some studies conducted on adults with newly-diagnosed ITP the use off-label of EPAG has also shown good results. However, there is still a significant proportion (about 20%) of pediatric patients who do not show a response to EPAG. Scarce data are currently available regarding the pharmacokinetics of EPAG in children: the estimated median plasma EPAG time to maximum plasma concentration (Tmax) and geometric mean plasma EPAG half-life (t1/2) values are consistent with the adult values, respectively 4 hours and 47-51 hours. The area under plasma concentration-time curve over a dosing interval at steady-state (AUC(0-t)) and maximum plasma concentration (Cmax) values were similar between adolescents and adults while children have higher dose normalized AUC(0-t) and Cmax.
Aims
Explore potential variability of EPAG pharmacokinetic in a cohort of pediatric patients affected by refractory ITP with no or variable response to EPAG or who experienced significant side effects.
Methods
Subjects aged 0 to 18 years were enrolled for pharmacokinetic assessment. Blood samples were obtained at 0, 2, 4 and 8 hours after the administration. Patients had started EPAG at least since 2 weeks. Analysis of EPAG plasma concentration was performed by mass spectrometry coupled with UHPLC (Ultra High Performance Liquid Chromatography) platform.
Results
We performed 40 PK analysis (24 Females, 16 Males). Patients who started therapy at different stages of the disease were considered (16 newly diagnosed, 12 persistent, 12 chronic). The median dose of EPAG given once daily was 50 mg, ranging from 12.5 mg (n subjects=3) to 150 mg (n subject=1). Overall, EPAG peak (Tmax) occured between 2 and 4 hours with a population Cmax of 50 ug/mL and t1/2 of 25 hours. Our cohort of patients therefore showed a Tmax consistent with the literature values, on the other hand, t1/2 appeared to be reduced when compared to known data. Moreover, 32% (13 patients) of our population had an extremely short half-life with values below 12 hours. With regard to exposure, 47.5% of patients (19 patients) have higher Cmax and AUC(0-t) values compared to literature. Based on these results, we tried to change the time of administration of EPAG in about 21 patients, who had shortened t1/2 and/or increased exposure, dividing the daily dose into 2 administrations. 15 patients showed a response, 10 showed a complete response (PLTs >100.000), 5 a partial response (PLTs 30.000-100.000), although 2 patients did not maintain the response in the follow-up and 1 had a fluctuating response.

Conclusion
These data highlight the need to further explore variability of EPAG exposure and its pharmacokinetic/pharmacodynamic profile in the pediatric population. Our results also suggest the possibility of improving the response to EPAG by modifying the administration although further studies are needed.
Keyword(s): Pediatric, Pharmacokinetic, Thrombocytopenia, Thrombopoietin (TPO)


